Targeting optimal blood pressure monitoring: what’s next?
The article “Optimal blood pressure during cardiopulmonary bypass defined by cerebral autoregulation” by Hori et al. (1) comes from a reputable group with extensive experience on intraoperative brain monitoring during cardiac surgery using continuous measurement of arterial pressure, cerebral blood velocity [transcranial Doppler (TCD)] and brain oxygenation [near-infrared spectroscopy (NIRS)] (2-7). Using the mean velocity index (Mx), a cerebral autoregulation index correlating mean arterial blood pressure (MAP) and TCD-derived middle cerebral artery (MCA) mean blood velocity, the authors sought to define the lower and upper limits of cerebral autoregulation (LLA and ULA, respectively) in 614 cardiac surgery patients undergoing cardiopulmonary bypass (CPB). They further tried to identify an “optimal-MAP” in this patient population, defining it as a MAP at which cerebral autoregulation was most performant (lowest Mx value). This current study is an extension of their previous work, incorporating patients from 3 specific cohorts.
Interestingly, they found that 17% of patients had an LLA above and 29% of patients had a ULA below the population “optimal-MAP” of 78 mmHg. This implies that adopting a one-size-fits-all approach of aiming a MAP of 78 mmHg would be inappropriate for 46% of patients by either under or overshooting MAP target. That is as good as flipping a coin! Furthermore, they found a strong association between the magnitude and duration of hypotension under a patient’s specific LLA with the risk of postoperative stroke. Their findings support the concept of tailoring blood pressure targets to a patient’s individual LLA to avoid severe postoperative complications.
The concept of an “optimal-MAP” as defined by the authors is novel in this patient population yet benefits of aiming such a MAP is uncertain. “Optimal-MAP” defined in the study is analogous to the “optimal-cerebral perfusion pressure (CPP)” previously defined in severe traumatic brain injury patients using the pressure reactivity index (PRx) as marker of cerebral autoregulation performance (8). Individualizing optimal-CPP in such patients has demonstrated potential clinical benefits (9). Brain injured patients are at high risk of intracranial hypertension and disturbance of blood-brain barrier, thus at considerable risk of cerebral hypoperfusion or hyperperfusion if CPP target is inadequate. In brain injured patients, identifying optimal-CPP where relative cerebral vasoconstriction is maximal allows balance between benefits of MAP augmentation on intracranial pressure (ICP) reduction while reducing risks of cerebral edema by transudation through leaky vessels. Contrarily to brain injured patients, it is conceptually unclear how “optimal-MAP” titration in cardiac surgery patients would confer benefit rather than simply aiming a MAP superior to the LLA of bilateral cerebral hemispheres to avoid hypoperfusion. A randomized trial currently carried out by the authors comparing cerebral autoregulation determined MAP versus standard care might bring further arguments to pursue this approach.
In this current study, the authors only reported findings from TCD-derived data. The same group had previously demonstrated that NIRS could represent an interesting alternative to TCD (2,10). Contrarily to NIRS, TCD-derived indices require a higher degree of expertise. Previous studies demonstrated a correlation between Mx and a similar NIRS-derived autoregulation index, the cerebral oximetry index (COx) and the tissue oximetry index (TOx) (2,4-6,11), in cardiac surgery as well as in non-cardiac surgery (12). However, Mx and COx are not directly interchangeable since determinant of mean velocity and cerebral oximetry remain quite different and measurements are taken in distinct vascular territories (MCA for TCD and anterior cerebral artery for NIRS). Despite absence of a true gold standard in continuous cerebral autoregulation monitoring, TCD-derived indices have been studied in a larger patient population. The reason why Mx was selected for this study rather than the simpler COx is unclear and unfortunately limits its clinical applicability in common practice. Developing a simple method in determining a given patient’s limits of cerebral autoregulation is necessary considering the wide inter-individual range of cerebral autoregulation in this patient population (3) and the authors’ work in this field is commendable. Nevertheless, several potential pitfalls related to the cerebral autoregulation assessment in this patient population must be considered.
First, the LLA as well as cerebral autoregulation performance in a given patient could vary during the surgical procedure due to changes in variables such as patient temperature (13), arterial partial pressure of carbon dioxide (14,15), the degree of hemodilution (15), and CPB flow rate and duration, as demonstrated by the authors in this study. Thus, target MAP might have to be modified during the case to ensure adequate cerebral perfusion. Also, intraoperative events, such as cerebral emboli (Figure 1), can occur before, during or after CPB and compromise brain perfusion.
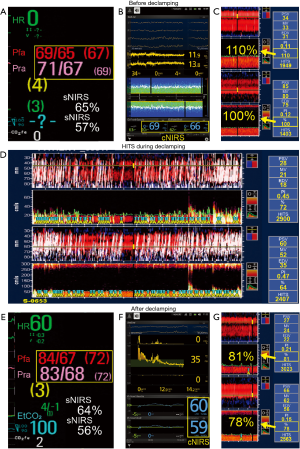
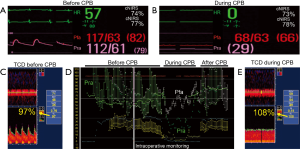
Second, erroneous assumptions in variables used in continuous monitoring of cerebral autoregulation could lead to false inferences. For example, errors in measuring arterial blood pressure could lead to inadequate conclusions on cerebral autoregulation performance or MAP thresholds. Up to 45% of patients undergoing CPB can develop a significant gradient between radial and femoral arterial pressure. Relying on radial artery measurements in such patients could skew cerebral autoregulation assessment and greatly influence the hemodynamic management (Figure 2) (16). Also, changes in ICP due to venous congestion (17) following superior vena cava compression or bicaval cannulation may unknowingly reduce CPP (CPP = MAP – ICP), affecting cerebral blood flow and cerebral autoregulation reserve. Untoward effects of vasoactive agents used intraoperatively such as phenylephrine and norepinephrine, are associated with a reduction in NIRS values (18,19). Independent effect of such commonly used vasoactive and inotropes agents on cerebral autoregulation indices requires further assessment.
Third, although taking cerebral autoregulation into consideration to guide blood pressure management during CPB is clinically attractive, however, one has to remember that there is no gold-standard cerebral autoregulation metric. In addition, there are multiple techniques to characterize static and dynamic cerebral autoregulation. The utilization of another method/metric in a comparable clinical situation (i.e., during CPB) could have led to different results (20).
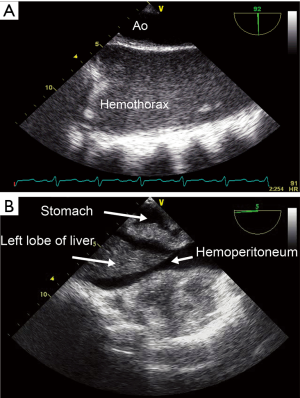
Finally, intraoperative cerebral monitoring could eventually challenge usual interventions currently used to correct hypotension or hypertension. For example, should clinicians similarly manage transient hypertension and hypotension during CPB considering the presence of hysteresis in the cerebral pressure-flow relationship? Indeed, recent findings support the notion that the human brain function is better adapted to react and compensate for physiologically-induced transient hypertension compared to transient hypotension, a notion not reflected in Lassen’s original cerebral autoregulation curve (21). Conversely, what should we do when blood pressure, TCD-derived blood velocity and NIRS-determined brain oxygenation drop? Should we correct it and how? A physiological approach using advanced monitoring allows identification of etiology and directed treatment. Reduced cerebral blood velocity and desaturation can result from an intracranial or an extracranial etiology. Using combined cerebral and somatic NIRS can help identifying the right etiology (22,23). Harmonious and parallel reductions in both cerebral and somatic NIRS values are suggestive of a non-cerebral etiology, such as cardiac dysfunction or bleeding, whereas isolated cerebral desaturation will usually indicate an intracranial cause (Figure 1). The use of processed electroencephalogram (pEEG) is also very useful when using TCD or NIRS. If NIRS-determined brain oxygenation decreases, TCD-derived cerebral blood velocity is normal and pEEG goes up, the brain desaturation could solely be secondary to the patient’s awakening for which increased sedation will correct it. However, if brain oxygenation decreases, along with cerebral blood velocity and pEEG, brain hypoperfusion is diagnosed. Modalities such as cardiac and extracardiac transesophageal echocardiography (TEE) (24,25) can also be useful in identifying the etiology of hypotension or reduction in NIRS-determined brain oxygenation during CPB. For instance, in patients on extra-corporeal membrane oxygenation following cardiac arrest, the reduction in brain oxygenation from acute anemia can be secondary to an undiagnosed occult hemothorax (Figure 3A) or hemoperitoneum easily diagnosed with TEE (Figure 3B).
In conclusion, cerebral autoregulation monitoring and a tailored approach to blood pressure targeting should be the ultimate goal in every patient undergoing cardiac surgery. But when things go wrong, what do we do next? Addressing correct etiology with proper intervention will be the key to improving outcome.
Acknowledgements
The authors would like to thank Mr. Denis Babin and Antoinette Paolitto for their help with the manuscript.
Footnote
Conflicts of Interest: Dr. Denault is on the speaker’s bureau for Masimo and CAE Healthcare. The other authors have no conflicts of interest to declare.
References
- Hori D, Nomura Y, Ono M, et al. Optimal blood pressure during cardiopulmonary bypass defined by cerebral autoregulation monitoring. J Thorac Cardiovasc Surg 2017;154:1590-8.e2. [Crossref] [PubMed]
- Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010;41:1951-6. [Crossref] [PubMed]
- Joshi B, Ono M, Brown C, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg 2012;114:503-10. [Crossref] [PubMed]
- Ono M, Joshi B, Brady K, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth 2012;109:391-8. [Crossref] [PubMed]
- Ono M, Arnaoutakis GJ, Fine DM, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013;41:464-71. [Crossref] [PubMed]
- Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014;147:483-9. [Crossref] [PubMed]
- Hori D, Ono M, Rappold TE, et al. Hypotension After Cardiac Operations Based on Autoregulation Monitoring Leads to Brain Cellular Injury. Ann Thorac Surg 2015;100:487-93. [Crossref] [PubMed]
- Lazaridis C, Smielewski P, Steiner LA, et al. Optimal cerebral perfusion pressure: are we ready for it? Neurol Res 2013;35:138-48. [Crossref] [PubMed]
- Dias C, Silva MJ, Pereira E, et al. Optimal Cerebral Perfusion Pressure Management at Bedside: A Single-Center Pilot Study. Neurocrit Care 2015;23:92-102. [Crossref] [PubMed]
- Ono M, Zheng Y, Joshi B, et al. Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesth Analg 2013;116:198-204. [Crossref] [PubMed]
- Hori D, Brown C, Ono M, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth 2014;113:1009-17. [Crossref] [PubMed]
- Chuan A, Short TG, Peng AZ, et al. Is cerebrovascular autoregulation associated with outcomes after major noncardiac surgery? A prospective observational pilot study. Acta Anaesthesiol Scand 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg 2010;110:321-8. [Crossref] [PubMed]
- Sanders RD, Degos V, Young WL. Cerebral perfusion under pressure: is the autoregulatory 'plateau' a level playing field for all? Anaesthesia 2011;66:968-72. [Crossref] [PubMed]
- Severdija EE, Vranken NP, Simons AP, et al. Hemodilution Combined With Hypercapnia Impairs Cerebral Autoregulation During Normothermic Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth 2015;29:1194-9. [Crossref] [PubMed]
- Fuda G, Denault A, Deschamps A, et al. Risk Factors Involved in Central-to-Radial Arterial Pressure Gradient During Cardiac Surgery. Anesth Analg 2016;122:624-32. [Crossref] [PubMed]
- Plochl W, Cook DJ, Orszulak TA, et al. Intracranial pressure and venous cannulation for cardiopulmonary bypass. Anesth Analg 1999;88:329-31. [PubMed]
- Nissen P, Brassard P, Jorgensen TB, et al. Phenylephrine but not ephedrine reduces frontal lobe oxygenation following anesthesia-induced hypotension. Neurocrit Care 2010;12:17-23. [Crossref] [PubMed]
- Brassard P, Pelletier C, Martin M, et al. Influence of norepinephrine and phenylephrine on frontal lobe oxygenation during cardiopulmonary bypass in patients with diabetes. J Cardiothorac Vasc Anesth 2014;28:608-17. [Crossref] [PubMed]
- Caldas JR, Haunton VJ, Panerai RB, et al. Cerebral autoregulation in cardiopulmonary bypass surgery: a systematic review. Interact Cardiovasc Thorac Surg 2018;26:494-503. [Crossref] [PubMed]
- Brassard P, Ferland-Dutil H, Smirl JD, et al. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 2017;312:H701-4. [Crossref] [PubMed]
- Lecluyse V, Couture EJ, Denault AY. A Proposed Approach to Cerebral and Somatic Desaturation in the Intensive Care Unit: Preliminary Experience and Review. J Cardiothorac Vasc Anesth 2017;31:1805-9. [Crossref] [PubMed]
- Hu T, Collin Y, Lapointe R, et al. Preliminary Experience in Combined Somatic and Cerebral Oximetry Monitoring in Liver Transplantation. J Cardiothorac Vasc Anesth 2018;32:73-84. [Crossref] [PubMed]
- Cavayas YA, Girard M, Desjardins G, et al. Transesophageal lung ultrasonography: a novel technique for investigating hypoxemia. Can J Anaesth 2016;63:1266-76. [Crossref] [PubMed]
- Denault AY, Beaubien-Souligny W, Elmi-Sarabi M, et al. Clinical Significance of Portal Hypertension Diagnosed With Bedside Ultrasound After Cardiac Surgery. Anesth Analg 2017;124:1109-15. [Crossref] [PubMed]


