A phase I/II study of bexarotene with carboplatin and weekly paclitaxel for the treatment of patients with advanced non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States with an estimated 222,500 new cases and 155,870 deaths in 2017 (1). Despite the progress in development of targeted treatments and immunotherapy, cytotoxic chemotherapy remains the backbone of many therapeutic regimens for advanced non-small cell lung cancer. Its use is associated with improved survival and symptom control (2). Yet many patients do not respond and eventually all patient progress. Better understanding of the molecular mechanisms of lung carcinogenesis is expected to lead to improved treatment outcomes. Retinoids and rexinoids are vitamin A derivatives implicated in cell reproduction, differentiation, growth and immune function (3,4). They act by altering gene expression mediated through two families of nuclear receptors: the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs). Retinoids bind to RARs, and rexinoids bind to RXRs (5). Retinoids and rexinoids induce degradation of cyclin D1 by ubiquitination and proteolysis in the proteosome, resulting in inhibition of cell growth (6). These in vitro observations have been confirmed in tissue analysis of tumors of early stage NSCLC patients treated with a rexinoid before resection (7). Amplification or overexpression of cyclin D1 plays a pivotal role in the development of numerous human cancers (8). In NSCLC, high cyclin D1 protein expression has been linked to shorter overall cancer-free survival (9).
Bexarotene is an oral rexinoid that is approved for the treatment of refractory early- and advanced-stage cutaneous T-cell lymphomas (10). Single-agent phase I studies demonstrated maximum tolerated doses ranging from 300 to 500 mg/m2/day. It has manageable toxicities including transiently elevated liver enzymes, leukopenia, hypertriglyceridemia (rarely leading to pancreatitis), and hypercalcemia. Investigations of bexarotene for treatment of NSCLC were initiated based on results from early bexarotene phase I trials showing disease stabilization in NSCLC patients (11,12). Early studies were encouraging as the addition of bexarotene enhanced the activity of several chemotherapeutic agents used in NSCLC, and prevented or overcame paclitaxel (13) and gemcitabine (14) resistance in NSCLC cell lines. It also showed promise in phase 2 trials when given with a platinum containing regimen as a first line treatment (15,16). An initial phase I/II study utilized full-dose cisplatin and vinorelbine with escalating doses of bexarotene and found 400 mg/m2 to be the optimal dose for phase II (15).
Based on the promising survival results from multiple phase I/II studies, we designed this trial to assess the safety and efficacy of bexarotene in a novel combination with carboplatin and a weekly paclitaxel schedule, chosen to provide better pharmacokinetic (PK) interaction and safety profile with daily bexarotene than could be accomplished with the typical once-every-three-week chemotherapy dosing. The primary endpoint of the study was to determine the safety of the combination, and the secondary endpoints were response rates, progression-free survival (PFS) and overall survival (OS).
Methods
We conducted a single-institution, open-label, single-arm dose-ranging trial of bexarotene with paclitaxel and carboplatin. Patients were enrolled at Dartmouth-Hitchcock Medical Center in Lebanon, NH, USA. Eligible patients were adults (>18 years) with histologically confirmed non-small cell lung cancer (clinical stage IIIB or IV, using AJCC v.5) who had Karnofsky performance status of 60% or greater, peripheral neuropathy < grade 2, adequate bone marrow, hepatic, thyroid and renal function studies documented within 14 days prior to study entry, fasting cholesterol ≤300 mg/dL, fasting triglycerides ≤400 mg/dL. Patients enrolled into the phase II component of this trial could not have received prior chemotherapy for their advanced NSCLC. Patients with severe gastrointestinal abnormalities including acute pancreatitis or active peptic ulcer disease and inability to tolerate oral medications were excluded. Patients with significant cardiac disease, myocardial infarction within the previous 3 months or serious cardiac arrhythmias were excluded. Patients with known hypersensitivity to Cremphor EL or retinoids were excluded.
The study was reviewed and approved by the Clinical Cancer Review Committee of the Norris Cotton Cancer Center and the Committee for the Protection of Human Subjects at Dartmouth College and conducted in accordance with the Declaration of Helsinki. All subjects were required to give informed consent for participation in this investigational trial. Study participation was monitored by the Norris Cotton Cancer Center Safety and Data Monitoring Committee following Dartmouth standard operating procedures.
Treatment plan
Paclitaxel at a dose of 100 mg/m2 was administered intravenously over 1 hour on days 1, 8, and 15 every 4 weeks. Carboplatin was given intravenously over 0.5 hours at a dose based upon the target area under the concentration versus time curve of 6 mg·min/mL (AUC =6) on day 1 every 4 weeks. Standard premedication regimens included steroids and anti-histamines. Bexarotene capsules were administered orally and given continuously beginning on the initial day of paclitaxel and carboplatin (day 1). In the phase I component of the study, two dose levels of bexarotene were studied (300 or 400 mg/m2/day). Anti-lipid therapy was started once elevated triglycerides were detected. In the phase II component of the study bexarotene was administered at the dose recommended during the phase I component. Patients were treated until disease progression, the occurrence of an unacceptable adverse event, or withdrawal of consent.
Bexarotene dose determination
Two dose levels of bexarotene (300 or 400 mg/m2) were studied in combination with carboplatin and weekly paclitaxel. At least 6 patients were entered onto each dose level. Intra-patient dose escalation was not allowed. An initial-dose-limiting toxicity (IDLT) was defined as a clinical observation that was attributable to the administration of bexarotene and necessitated a reduction in dose, suspension, or discontinuation of study drug because of grade 3 or 4 toxicity. The recommended phase II dose was defined as the highest dose of bexarotene (300 or 400 mg/m2) in combination with carboplatin and weekly paclitaxel that induced IDLT in fewer than or equal to 33% of patients.
Statistical plan
The primary aim for this study was to evaluate the safety of administering daily oral bexarotene at two dose levels (300 or 400 mg/m2) in combination with carboplatin and weekly paclitaxel in patients with stage IIIB or IV NSCLC. A secondary aim was to evaluate the efficacy, as measured by response rates using RECIST 1.0, and to assess overall and PFS. A true response rate of 20% or greater was considered sufficient to warrant further investigation. A true response rate of 10% or less indicated that the combination had limited activity and did not warrant further study. A two-stage sequential design was used to permit early termination of the study. The design called for an initial enrollment of 20 patients during the first stage of the study. If no patients out of the initial 20 responded, the study would be terminated because the true response rate would not meet the above criteria. If more than 6 patients out of the first 20 patients responded, the study would be terminated and consideration would be given to proceeding with a randomized phase III study. If observed responses were between 1 and 6 patients, the study would continue to the second stage. Upon completion of the study the true response rate was estimated using the observed response rate, and an exact confidence interval was constructed, according to the method described by Jennison and Turnbull (17).
Results
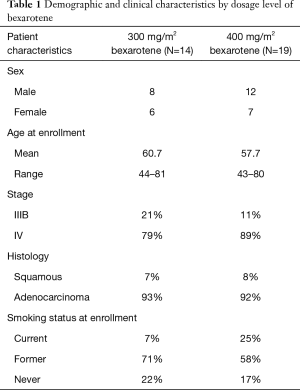
Thirty-three patients were enrolled in this study between January 1, 2002 and December 2, 2004 (Table 1). Thirteen patients (39%) were female. The majority of patients in both dosage groups of bexarotene had stage IV NSCLC (11/14 in 300 mg/m2 group, 17/19 in 400 mg/m2 group). The average age of patients was 61 (range, 44–81) years in 300 mg/m2 group, and 58 (range, 43–80) years in 400 mg/m2 group. The majority of patients in both groups were either former or current smokers (78% and 83% in the 300 and 400 mg/m2 groups, respectively).
Full table
Safety
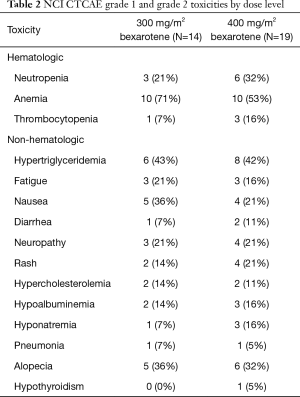
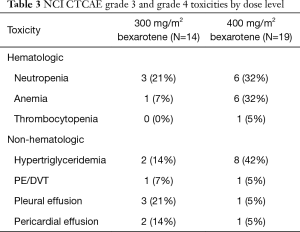
All patients were evaluated for toxicity. Bexarotene was well tolerated at either dose (300 or 400 mg/m2) when added to chemotherapy with carboplatin and weekly paclitaxel. The frequency and distribution of grade 1 and 2 toxicities regardless of relatedness to treatment are presented in Table 2. One DLT developed at each dose level. Myelosuppression was more common in the 400 mg/m2 dosage group. The frequency of distribution of grade 3 and 4 toxicities is shown in Table 3. Hypertriglyceridemia was commonly seen (8/14, 57% in 300 mg/m2 group), and (16/19, 84% in 400 mg/m2 group) . There was no evidence of enhancement in toxicity with this combination regimen, and toxicities were as would be expected from the chemotherapeutic agents or bexarotene alone. Based on the generally low frequency of DLTs, bexarotene at 400 mg/m2 was recommended for further study in phase II.
Full table
Full table
Efficacy
There were 6 partial responders among the first 20 patients and the study proceeded to full enrollment of 33 patients. Of these 33 patients, 31 were evaluable for response and 11 had a partial response to therapy, for a 35% response rate, 95% CI (19–55%). Median progression free survival was 4.8 months (1–63 months). OS was 8.3 months (1–167+ months). There was no significant difference in PFS or OS between patients treated at the different bexarotene dosages (PFS HR, 0.84, Log rank P=0.61; OS HR, 0.73, Log rank P=0.37).
Effect of hypertriglyceridemia on efficacy
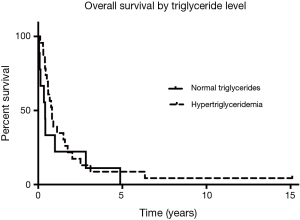
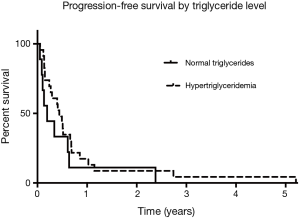
Since the start of the trial, evidence emerged for hypertriglyceridemia as a marker for bexarotene clinical activity. There was one responder among the patients with triglycerides <200 mg/mL and 10 responses among the patients with triglycerides >200 mg/mL. Among the subset of patients who experienced hypertriglyceridemia at the 6-week follow-up visit, OS was 9.8 months, compared to the patients who did not develop hypertriglyceridemia (4.9 months) as shown in Figure 1 (HR, 0.69; Log rank P=0.33). Median PFS was 5.3 months for the patients who had hypertriglyceridemia and 2.3 months for the patients without elevated triglycerides as shown in Figure 2 (HR, 0.64; Log rank P=0.24). There was no significant difference in PFS between those with dose held or lowered for side effects and those without.
Discussion
This study expands the current experience with bexarotene in combination with chemotherapy. A weekly paclitaxel schedule that has not been evaluated previously in combination with bexarotene, was utilized in order to enhance PK interactions. In contrast to other bexarotene studies where antilipid therapy was initiated from day 1, we did not start atorvastatin unless hypertriglyceridemia developed. Such design allowed for clearer assessment of the activity and toxicity of the combination, without the confounding effect of atorvastatin. We found response rates (35%) and survival (8.3 months) that were numerically higher but generally within the range expected for chemotherapy alone. Bexarotene addition to already studied dosages of carboplatin and paclitaxel appeared to be well tolerated in our study. Except for hypertriglyceridemia that was easy to manage, the incidence of toxicities was no greater than that previously shown in patients receiving combination chemotherapy with carboplatin and paclitaxel alone. There were no new or unexpected toxicities identified in this study. Known toxicities of carboplatin and paclitaxel including alopecia, neuropathy, anemia, and neutropenia were observed, but the incidence of these toxicities did not appear to be greater than that expected in patients who receive the same combination chemotherapy without bexarotene (18). As expected, hypertriglyceridemia was frequent and of the same magnitude as expected when bexarotene is used as a single agent.
There did appear to be a survival benefit in a subset of patients in our population—those who experienced bexarotene-induced hypertriglyceridemia. There was a clinically meaningful increase in OS (an increase in median survival from 4.9 to 9.8 months) and in PFS (an increase from 2.3 to 5.3 months) for the patients with any degree of hypertriglyceridemia, compared to those who had normal triglyceride levels. However, survival was not statistically different between the patients who suffered from hypertriglyceridemia to the point that doses of bexarotene were held or reduced. This PFS of 4.8 months and OS of 8.3 months was similar to the survival of those patients treated with the same regimen of carboplatin and paclitaxel alone [4.6 and 9.6 months for PFS and OS, respectively (18)]. We have previously reported such association between survival and hypertriglyceridemia in a study of bexarotene and erlotinib (19).
Two large randomized trials of bexarotene with cisplatin/vinorelbine (SPIRIT I) and with a different schedule of carboplatin/paclitaxel (SPIRIT II) were published since the start of our study (20,21). Our results are consistent with their findings of no survival benefit from adding bexarotene to chemotherapy. PK evaluation of drug-drug interactions between bexarotene and the chemotherapy agents determined that paclitaxel, free carboplatin, and total carboplatin concentrations were similar with or without bexarotene (22). Co-administration of chemotherapy did, however, alter bexarotene PK as both the Cmax and AUC of bexarotene were significantly increased with concomitant treatment with paclitaxel and carboplatin (22). Based on the results of this PK study, it is unlikely that a PK drug interaction between bexarotene and carboplatin or paclitaxel would explain the lack of significantly improved efficacy of this combination.
In both SPIRIT trials and in a bexarotene monotherapy trial in NSCLC patients (23), survival improved in patients who experienced triglyceride elevations. Notably, OS rates of greater than 12 months in patients with bexarotene-induced hypertriglyceridemia are among the highest reported for unselected patients with stage IV NSCLC. In contrast to these trials where atorvastatin was started with cycle one, in our study anti-lipid therapy was initiated only after hypertriglyceridemia was detected, yet a similar association with improved survival was noted, indicating that elevated triglycerides may be an important predictive factor. The mechanism by which bexarotene induces hypertriglyceridemia is known. The survival benefit in the patients with bexarotene induced hypertriglyceridemia raises the question of how this might help more aggressively target such tumor sensitivity. Our trial was designed before molecular testing of tumors became available. Several studies evaluated the activity of bexarotene in combination with targeted drugs such as erlotinib (19,24), gefitinib (25), and rosiglitazone (26). We showed that the bexarotene/erlotinib regimen was active in NSCLC patients with K-ras mutations and without activating EGFR mutations. Cyclin D1 emerged as a biomarker of response to the combination. Our results with this regimen were confirmed by the BATTLE trial (27). Since the completion of our trial, immunotherapy has shown impressive activity in various cancers, including NSCLC, either in first-line or in subsequent lines of treatment (28,29). There is emerging evidence for immunomodulating effects of retinoids and rexinoids (30,31). While bexarotene should not be further studied in combination with chemotherapy in unselected patients with NSCLC, further explorations of the mechanisms underlying the observed benefit in defined subsets, such as patients with hypertriglyceridemia or molecularly selected patients, and in combination with immunotherapy, are warranted.
Acknowledgements
This works was supported by Ligand Pharmaceuticals who provided the study drug and funding for the conduct of this study.
Footnote
Conflicts of Interest: KH Dragnev and JR Rigas received funding from Ligand Pharmaceuticals for the conduct of this study. Ligand Pharmaceuticals had no role in the data collection, analysis, interpretation or composition of the manuscript. The other authors have no conflicts of interest to declare.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Schiller JH, Harrington D, Sandler A, et al. A randomized phase III trial of four chemotherapy regimens in advanced non-small cell lung cancer (NSCLC). Proceedings of ASCO 2000;19.
- Hong WK, Itri LM. Retinoids and human cancer. In: Sporn MB, Roberts AB, Goodman DS. editors. The retinoids: biology, chemistry, and medicine. Second ed. New York, NY: Raven Press Ltd., 1994:597-630.
- Gudas LJ, Sporn MB, Roberts AB. Cellular biology and biochemistry of retinoids. In: Sporn MB, Roberts AB, Goodman DS. editors. The retinoids: biology, chemistry, and medicine. Second ed. New York, NY: Raven Press Ltd., 1994:443.
- Wei LN. Retinoid receptors and their coregulators. Annu Rev Pharmacol Toxicol 2003;43:47-72. [Crossref] [PubMed]
- Dragnev KH, Freemantle SJ, Spinella MJ, et al. Cyclin proteolysis as a retinoid cancer prevention mechanism. Ann N Y Acad Sci 2001;952:13-22. [Crossref] [PubMed]
- Dragnev KH, Petty WJ, Shah SJ, et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res 2007;13:1794-800. [Crossref] [PubMed]
- Fu M, Wang C, Li Z, et al. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology 2004;145:5439-47. [Crossref] [PubMed]
- Keum JS, Kong G, Yang SC, et al. Cyclin D1 overexpression is an indicator of poor prognosis in resectable non-small cell lung cancer. Br J Cancer 1999;81:127-32. [Crossref] [PubMed]
- Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced- stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 2001;19:2456-71. [Crossref] [PubMed]
- Miller VA, Benedetti FM, Rigas JR, et al. Initial clinical trial of a selective retinoid X receptor ligand, LGD1069. J Clin Oncol 1997;15:790-5. [Crossref] [PubMed]
- Rizvi NA, Marshall JL, Dahut W, et al. A Phase I study of LGD1069 in adults with advanced cancer. Clin Cancer Res 1999;5:1658-64. [PubMed]
- Yen WC, Corpuz MR, Prudente RY, et al. A selective retinoid X receptor agonist bexarotene (Targretin) prevents and overcomes acquired paclitaxel (Taxol) resistance in human non-small cell lung cancer. Clin Cancer Res 2004;10:8656-64. [Crossref] [PubMed]
- Tooker P, Yen WC, Ng SC, et al. Bexarotene (LGD1069, Targretin), a selective retinoid X receptor agonist, prevents and reverses gemcitabine resistance in NSCLC cells by modulating gene amplification. Cancer Res 2007;67:4425-33. [Crossref] [PubMed]
- Khuri FR, Rigas JR, Figlin RA, et al. Multi-institutional phase I/II trial of oral bexarotene in combination with cisplatin and vinorelbine in previously untreated patients with advanced non-small-cell lung cancer. J Clin Oncol 2001;19:2626-37. [Crossref] [PubMed]
- Edelman MJ, Smith R, Hausner P, et al. Phase II trial of the novel retinoid, bexarotene, and gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5774-8. [Crossref] [PubMed]
- Jennison C, Turnbull BW. Repeated confidence intervals for group sequential clinical trials. Control Clin Trials 1984;5:33-45. [Crossref] [PubMed]
- Belani CP, Ramalingam S, Perry MC, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol 2008;26:468-73. [Crossref] [PubMed]
- Dragnev KH, Ma T, Cyrus J, et al. Bexarotene plus erlotinib suppress lung carcinogenesis independent of KRAS mutations in two clinical trials and transgenic models. Cancer Prev Res (Phila) 2011;4:818-28. [Crossref] [PubMed]
- Blumenshein G, Khuri FR, Gatzemeier U, et al. A randomized phase III trial comparing bexarotene/carboplatin/paclitaxel versus carboplatin/paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small cell lung cancer (NSCLC). Proc ASCO 2005;23.
- Ramlau R, Zatloukal P, Jassem J, et al. Randomized phase III trial comparing bexarotene (L1069-49)/cisplatin/vinorelbine with cisplatin/vinorelbine in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT I. J Clin Oncol 2008;26:1886-92. [Crossref] [PubMed]
- Rodon J, Jacobs CD, Chu Q, et al. A phase I pharmacokinetic study of bexarotene with paclitaxel and carboplatin in patients with advanced non-small cell lung cancer (NSCLC). Cancer Chemother Pharmacol 2012;69:825-34. [Crossref] [PubMed]
- Govindan R, Crowley J, Schwartzberg L, et al. Phase II trial of bexarotene capsules in patients with advanced non-small-cell lung cancer after failure of two or more previous therapies. J Clin Oncol 2006;24:4848-54. [Crossref] [PubMed]
- Dragnev KH, Petty WJ, Shah S, et al. Bexarotene and erlotinib for aerodigestive tract cancer. J Clin Oncol 2005;23:8757-64. [Crossref] [PubMed]
- Padda SK, Chhatwani L, Zhou L, et al. Phase I and pharmacokinetic study of bexarotene in combination with gefitinib in the third-line treatment of non-small-cell lung cancer: brief report. Anticancer Drugs 2013;24:731-5. [Crossref] [PubMed]
- Read WL, Baggstrom MQ, Fracasso PM, et al. A phase I study of bexarotene and rosiglitazone in patients with refractory cancers. Chemotherapy 2008;54:236-41. [Crossref] [PubMed]
- Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov 2011;1:44-53. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. New Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Takeuchi H, Yokota-Nakatsuma A, Ohoka Y, et al. Retinoid X Receptor Agonists Modulate Foxp3(+) Regulatory T Cell and Th17 Cell Differentiation with Differential Dependence on Retinoic Acid Receptor Activation. J Immunol 2013;191:3725-33. [Crossref] [PubMed]
- Chandraratna RA, Noelle RJ, Nowak EC. Treatment with retinoid X receptor agonist IRX4204 ameliorates experimental autoimmune encephalomyelitis. Am J Transl Res 2016;8:1016-26. [PubMed]


