Application of the “continuous suture dividing and equal suture tightening” method in video-assisted thoracoscopic surgery sleeve lobectomy
Introduction
Roviaro et al. (1) reported the first video-assisted thoracoscopic surgery (VATS) lobectomy for lung cancer in 1992; since then, the technique has developed rapidly and has been applied widely in lung surgery. The method has been shown to be safe, minimally invasive, and radical (2,3). Currently, VATS lobectomy is the major treatment of early-stage non-small cell lung cancer (NSCLC) (4). When the tumor is located in the orifice of the lobe bronchus or invades the main bronchus, bronchial sleeve lobectomy is required. However, since it is a challenging and complicated process for bronchial anastomosis, VATS sleeve lobectomy is usually converted to open thoracotomy (5,6). In 2002, Santambrogio et al. reported the first case of a complete VATS bronchial sleeve lobectomy for the treatment of mucoepidermoid carcinoma of the left lower lobe (7). Mahtabifard et al. reported about VATS sleeve lobectomy in 13 patients in 2008; the advantages of the method include small incisions and a short length of hospital stay with acceptable morbidity and mortality (6). However, VATS sleeve lobectomy is rarely reported, as it is a complicated and challenging process for bronchial anastomosis (8). The anastomosis technique in thoracoscopy might differ from that in thoracotomy, owing to the confined costal space for surgical access and the limited visual monitoring of the suture threads. Therefore, entanglement of the suture threads can be a major issue while performing interrupted or continuous suturing. Thus, tightening the suture to avoid suture entanglement is a key point of the operation. Relevant reports on the tightening suture method in bronchial anastomosis under thoracoscopy are available. Thus, we developed a feasible and secure tightening suture method for bronchial anastomosis in VATS sleeve lobectomy. Herein, we report our clinical experience of VATS sleeve lobectomy using the “continuous suture dividing and equal suture tightening” method in 17 patients.
Clinical data
Between November 2013 and April 2015, 17 VATS sleeve lobectomies were performed at Tongji Hospital; the cohort consisted of 14 males and 3 females, with a median age of 52.8±13.9 (range, 26–68) years. Patients underwent VATS sleeve lobectomy at the following locations: right upper lobe (n=11), left lower lobe (n=3), and left upper lobe (n=3). Table 1 summarizes the patient characteristics.
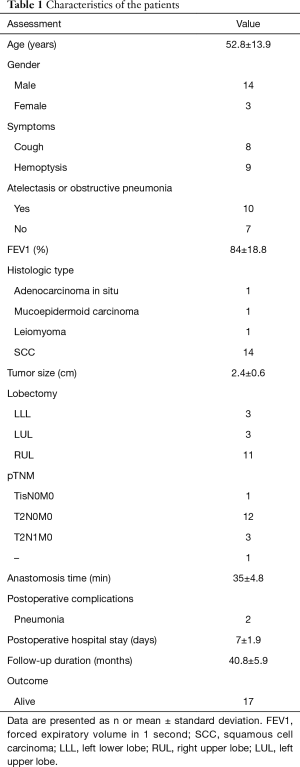
Full table
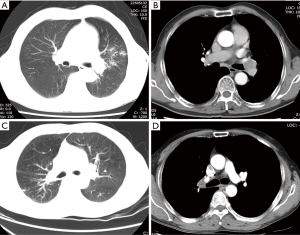
All patients were preoperatively evaluated with enhanced chest computed tomography (CT) and bronchoscopy. Enhanced chest CT was used to confirm the tumor location, size, and the absence of vascular invasion (Figure 1). Chest CT could also detect the absence of mediastinal lymph node enlargement. Bronchoscopy confirmed the tumor location and degree of airway invasion. If the tumor was located at the lobe orifice or invaded the adjacent main bronchus, it was considered suitable for bronchoplasty. The pathological classification was confirmed by a bronchoscope biopsy. Preoperative brain CT, bone scan, abdominal ultrasonography, or a positron emission tomography-computed tomography (PET-CT) scan was used to exclude metastasis. Preoperative studies and lab results were not notable except in cases with tumors. The study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-C20131011). An informed consent was obtained before beginning the study.
Methods
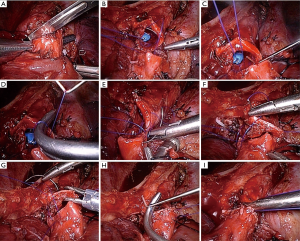
All cases received intravenous anesthesia and inhalation anesthesia through a double-lumen endotracheal intubation. Patients were placed in the lateral decubitus position. A 4-port VATS technique was used, and a camera port was made in the 8th intercostal space, along the median axillary line. The utility operation ports were made in the 4th and 6th intercostal space, anterior axillary line, without rib spreading. An additional port was made in the 7th intercostal space on the scapular line for the assistant. The port at the 4th intercostal space was approximately 2.5 cm long, while the other ports were approximately 1.5 cm in length. The trocar was introduced only through the camera port. The surgeon and the second assistant stood on the abdominal side of the patient, while the first assistant stood behind the patient. An endoscopic linear stapler was used to cut off the fissure and pulmonary vessels separately (Figure 2). After radical systematic dissection of lymph nodes in all cases with a malignant disease similar to that in routine lobectomy, we divided the bronchus of the target lobe with a minimum of 0.5 cm proximal margin. A hilar vascular clamp was placed below the bronchus to be removed in order to function as the support protecting the tissue below the bronchi. Bronchial margins were subjected to frozen section pathological analysis, which confirmed the free margins. Bronchial anastomosis was performed in an end-to-end fashion using a Surgipro II monofilament polypropylene 4-0 (Covidien, MA, USA). Then, we performed bronchial anastomosis using the “continuous suture dividing and equal suture tightening” method.

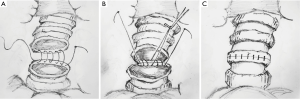
The anastomosis was divided into three or four equal portions for suturing. From the starting point to the end of each portion, tightening of the suture was not required, and the front section of the suture did not need to be extremely long. While suturing, only the length of the suture required for the next needle is required. Consequently, suturing of each equal portion suture was completed using nerve retractors and hilum forceps to tighten the sutures in order to ensure the necessary tension. This method was completed for all of the equal portions as follows (Figure 3): the needle holder was passed through the main access port, beginning with the deepest portion. For suturing of the deepest portion, closure was begun with a 4-0 monofilament polypropylene (Surgipro Covidien) suture using a needle from the membrane-cartilage junction from outside to inside. The front section of the suture should be sufficiently long for stitching. If the front suture is long, it is easy to twist. The deepest portion of the cartilaginous portion was made to be the lateral end using a continuous suture (Figure 4A). The nerve retractor and hilum forceps used for pulling the suture needles were the same length. Then, the nerve retractor and hilum forceps were used to tighten the suture of the deepest portion (Figures 4B,4C,5). Subsequently, the continuous sutures of the other portions were finished, and the nerve retractor and hilum forceps were used to tighten the sutures after suturing each portion (Figure 6). Finally, the remaining bronchial sutures were placed, and ligation was completed at the lateral end. Intraoperative bronchoscopy was performed to clear the airways of blood and secretions. The anastomosis was checked for an air leak in the presence of water by inflating the lungs. The pedicled aortic adventitial flap was applied to reinforce the closure of the bronchial anastomotic stoma in the left VATS sleeve lobectomy (12) along with the pedicled azygos vein flap in right VATS sleeve lobectomy. The chest tube was placed, and the incisions were closed.


Postoperative management
Postoperative management of the patients who underwent VATS sleeve lobectomy was similar to those of patients who underwent VATS lobectomy. It mainly included the prophylactic use of antibiotics, postoperative analgesia, and airway management. In addition, nutrition supplementation, observation of air bubble escape and the color of chest tube drainage, and effective expectoration should also be encouraged. One day after the operation, bronchoscopy was used to assess the anastomosis and the inspiration of sputum. A chest X-ray was used to evaluate the recruitment of the residual lung. The chest tube was removed when drainage was <200 mL/day. One week after the surgery, a CT three-dimensional (3D) reconstruction and bronchoscopy were used to monitor the anastomosis. A 3-month follow-up consisting of bronchoscopy and CT scans were conducted to observe the recurrence and stenosis of the anastomosis.
Results
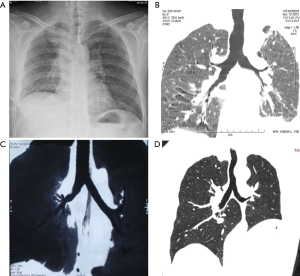
Herein, we described 17 cases of VATS sleeve lobectomy with bronchial anastomosis using the “continuous suture dividing and equal suture tightening” method. Eleven patients underwent upper right sleeve lobectomy, 3 patients underwent upper left sleeve lobectomy, and 3 patients underwent lower left sleeve lobectomy. All of the operative procedures were uneventful with no unplanned conversions to thoracotomy. The bronchial anastomoses were successful in all cases involving the “continuous suture dividing and equal suture tightening” method. The median bronchial anastomosis time was 35±4.8 (range, 30–45) min. The chest tubes were removed after a median time of 3 (range, 2–5) days, and the length of the hospital stay was in median 7±1.9 (range, 5–10) days. Pathologically, 14 cases presented squamous cell carcinomas, 1 adenocarcinoma in situ, 1 mucoepidermoid carcinoma, and 1 leiomyoma (1 TisN0M0, 12 T2N0M0, and 3 T2N1M0, respectively). Bronchial margins were negative in all cases. Approximately 1 week postoperatively, CT and bronchoscopy showed that the anastomotic stoma healed satisfactorily. However, no operative mortality or bronchial pleural fistula were observed. One patient presented pneumonia postoperatively, but was successfully treated with antibiotic therapy. All patients survived and were postoperatively followed for an average of 40.8±5.9 months. Bronchoscopy or 3D-CT were performed to confirm the absence of local recurrence and stenosis (Figure 7).
Discussion
With the development of minimally invasive surgery techniques, the VATS method has been increasingly applied for the treatment of lung cancer patients. Currently, total thoracoscopic lobectomy is performed worldwide (2,3,13). However, tumor or metastatic lymph node invasion in the orifice of the bronchi has long been regarded as a contraindication for VATS lobectomy (6). Reportedly, bronchial sleeve lobectomy for partial central lung cancer allows resection of an endobronchial lesion with maximal conservation of lung parenchyma (14,15). Therefore, bronchial sleeve lobectomy is a standard procedure for central lung cancer. Until recently, sleeve resection was viewed as an absolute contraindication for VATS lobectomy, despite the numerous advantages associated with minimally invasive procedures. In addition, the trauma caused by thoracotomy severely affects the quality of life of patients after the operation and increases perioperative mortality (6,15). However, VATS sleeve lobectomy is rarely reported. Santambrogio et al. reported the world’s first VATS sleeve lobectomy with bronchoplasty in a 15-year-old female with a low-grade mucoepidermoid carcinoma of the left lower lobe of the bronchus (7). In recent years, the efficacy and safety of VATS sleeve lobectomy has been reported in select cases (6,16-19). Nevertheless, it is a safe and feasible technique used by skilled thoracic surgeons to complete bronchial anastomosis in appropriate cases (6,19). The successful development of VATS sleeve lobectomy has been helpful for promoting minimally invasive surgeries and for expanding the indications for thoracoscopic surgeries in lung cancer patients. This method can not only avoid pneumonectomy but also keeps the lung parenchyma healthy in order to improve patient quality of life after lung tumor resection. However, only a few medical centers can carry out this complicated thoracoscopic surgery; the main difference between VATS lobectomy and VATS sleeve lobectomy is the need for bronchial anastomosis. VATS anastomosis of the bronchus is difficult and requires not only a thoracoscopic lobectomy and sleeve lobectomy but also a skilled suturing technique during thoracoscopy. The key and difficult point of the VATS sleeve lobectomy is the anastomosis of the bronchi (8,20). Furthermore, thoracoscopic bronchial anastomosis differs from conventional open surgeries. All operations are performed in an intrathoracic mode with a thoracoscope, and tightening of the suture is challenging, which is especially critical in thoracoscopic surgeries, wherein the surgeons have a limited operative view and narrow working space. Since the thoracoscope can view sutures only partially, the intertwining of the sutures poses a challenge in bronchial anastomosis.
There are two main kinds of bronchial anastomosis: interrupted suture and continuous suture. Mahtabifard et al. reported 13 cases of thoracoscopic bronchial sleeve lobectomy with absorbable sutures via the main operating hole for interrupted suture bronchial anastomosis (6). Since the knot of the bronchial suture of the posterior bronchial wall was located in the bronchial cavity, the development of a postoperative cough and other complications increased. Monitor-based visualization restricts the field of view, and extraluminal ligations of the anastomosis of the deep part of the bronchus are difficult to perform. Entanglement of the suture threads is a major concern while performing interrupted suturing, and this technique requires supplementary time for organization. However, the placement of interrupted sutures can be a complex and time-consuming process. Lunxu et al. reported bronchial anastomosis performed with a running suture using a 3–0 Prolene stitch by a long endoscopic needle holder through the assistant incision (21). However, the use of this kind of suture in surgeries in the future is unknown, and the method used to tighten the sutures is a key factor in this kind of operation. Mishandling easily results in anastomotic stricture or partial omission. Ohata et al. reported that the main right bronchus and truncus intermedius have been anastomosed by continuous sutures with two 3-0 Vicryl (Ethicon) threads (20). This study did not address the key techniques necessary to avoid entanglement of the sutures or the way the sutures were tightened. The tightening of the suture can pose a challenge and may lead to anastomotic leakage or dehiscence.
Entanglement of the sutures is difficult to avoid, irrespective of the use of interrupted or continuous anastomosis under thoracoscopy, which greatly affects the anastomosis process. The method used for tightening sutures in order to avoid interference as a result of suture entanglement is the key to this operation. Several studies suggested that the results of the continuous suturing technique are similar to those of the interrupted suturing technique in cases of bronchial sleeve resections. Moreover, continuous suturing is simpler, faster, and more economical compared to interrupted suturing (22,23). Thus, herein, we adopted a method of continuous suturing. To the best of our knowledge, this is the first study to use the “continuous suture dividing and equal suture tightening” method in VATS sleeve lobectomy for bronchial anastomosis. No suture interference was observed, suture entanglement was avoided, and the anastomoses were completed smoothly. Each part was completed using a nerve hook and hilar vessel forceps to tighten the sutures. Interestingly, the incidence of anastomotic fistulae was reduced, and the size of the stitches in each section was controlled, thereby effectively avoiding the occurrence of anastomotic strictures. The advantage of this method for bronchial anastomosis is its technical simplicity and reduced operative time.
A major limitation of this study is the low number of patients treated with this technique. We plan on expanding the number of patients to truly evaluate the feasibility of this technique. Furthermore, based on our experience, the indications for VATS sleeve lobectomy are a central NSCLC with a diameter of no more than 3 cm, lack of vascular invasion, lack of invasion to the trachea and carina. This technique should be used to determine more specific criteria for VATS sleeve lobectomy in patients. This method has been shown to be more apt at correctly recommending VATS sleeve lobectomy in select patients with central lung cancer.
This is the first study to use the “continuous suture dividing and equal suture tightening” method for bronchial anastomosis in VATS sleeve lobectomy; moreover, the method is feasible and secure. We confirmed that this suturing method is simple and effective at avoiding suture entanglement, thereby enabling the widespread adoption of VATS sleeve lobectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-C20131011). An informed consent was obtained before beginning the study.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Hanna JM, Berry MF, D'Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5 Suppl 3:S182-9. [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [Crossref] [PubMed]
- Zhang Z, Huang Q, Liao Y, et al. An endoscopic linear stapler was used to cut off the fissure and pulmonary vessels separately. Asvide 2018;5:773. Available online: http://www.asvide.com/article/view/27388
- Zhang Z, Huang Q, Liao Y, et al. “Continuous suture dividing and equal suture tightening” of the deepest portion. Asvide 2018;5:774. Available online: http://www.asvide.com/article/view/27389
- Zhang Z, Huang Q, Liao Y, et al. “Continuous suture dividing and equal suture tightening” of the other portions was completed. Asvide 2018;5:775. Available online: http://www.asvide.com/article/view/27391
- Jiang WY, Liao YD, Cai YX, et al. Application of pedicled aortic adventitia flap in the reinforcement of bronchial stump or bronchial anastomotic stoma closure in left pulmonary resection. J Thorac Cardiovasc Surg 2014;148:351-3. [Crossref] [PubMed]
- Chen H, Huang L, Xu G, et al. Modified bronchial anastomosis in video-assisted thoracoscopic sleeve lobectomy: a report of 32 cases. J Thorac Dis 2016;8:2233-40. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Eichhorn F, Storz K, Hoffmann H, et al. Sleeve pneumonectomy for central non-small cell lung cancer: indications, complications, and survival. Ann Thorac Surg 2013;96:253-8. [Crossref] [PubMed]
- Yu D, Han Y, Zhou S, et al. Video-assisted thoracic bronchial sleeve lobectomy with bronchoplasty for treatment of lung cancer confined to a single lung lobe: a case series of Chinese patients. J Cardiothorac Surg 2014;9:67. [Crossref] [PubMed]
- Xu X, Chen H, Yin W, et al. Thoracoscopic half carina resection and bronchial sleeve resection for central lung cancer. Surg Innov 2014;21:481-6. [Crossref] [PubMed]
- Xu G, Zheng W, Guo Z, et al. Complete video-assisted thoracoscopic surgery upper left bronchial sleeve lobectomy. J Thorac Dis 2013;5 Suppl 3:S298-300. [PubMed]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Ohata K, Zhang J, Ito S, et al. Thoracoscopic Bronchoplasty Using Continuous Sutures in Complete Monitor View. Ann Thorac Surg 2014;98:1132-3. [Crossref] [PubMed]
- Liu L, Mei J, Pu Q, et al. Thoracoscopic bronchovascular double sleeve lobectomy for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;46:493-5. [Crossref] [PubMed]
- Bayram AS, Erol MM, Salci H, et al. Basic interrupted versus continuous suturing techniques in bronchial anastomosis following sleeve lobectomy in dogs. Eur J Cardiothorac Surg 2007;32:852-4. [Crossref] [PubMed]
- Kutlu CA, Goldstraw P. Tracheobronchial sleeve resection with the use of a continuous anastomosis: results of one hundred consecutive cases. J Thorac Cardiovasc Surg 1999;117:1112-7. [Crossref] [PubMed]


