Lung function changes after chemoradiation therapy in patients with lung cancer treated by three usual platinum combinations
Introduction
Lung cancer is a leading cause of cancer deaths worldwide. Approximately 1.2 million people die from lung cancer each year. The prognosis for lung cancer patients is highly dependent on the stage at diagnosis, and the high death rate is due to the fact that lung cancer is usually in an advanced stage when first diagnosed and only 15% of lung cancers are discovered in the early localized stage (1). Patients with unresectable lung tumors and with good performance status (PS) receive platinum-based chemotherapy, but at the cost of potentially severe toxicity. The lung is a common target of chemotherapy toxicity, and a variety of treatments have been recognized (2) as causative agents in lung injury. Pulmonary function tests (PFTs) provide the opportunity for detection of drug-associated pulmonary toxicity (2), determining the presence and extent of damage (3).
Radiation therapy may also cause lung parenchyma injury in form of acute radiation pneumonitis or late fibrosis in patients with lung carcinoma as well as other primaries where the lung might be irradiated such as breast carcinomas or lymphomas (4,5). Several patient-specific factors such as gender, age, smoking history, tumor location and performance score have also been proposed as potential predictors of the risk of radiation pneumonitis, but these have not been consistently demonstrated across different studies. The risk of radiation pneumonitis also seems to increase as the cumulative dose of radiation to normal lung tissue increases, as measured by dose-volume histograms (6). Treatment-specific factors associated to radiation therapy, such as chemotherapy regimen and doses may also be a factor of lung function impairment and complications (7). Yet, several studies demonstrate that the addition of chemotherapy to RT improves patients’ survival in stage IIIB non-small-cell (NSCLC) (8) and limited to the thorax small-cell lung carcinomas (SCLC) (9) at the expense of more acute local toxicity.
Although lung toxicity of chemoradiation is well known, no prospective study reported results on lung function comparing directly the different platinum combinations associated to radiation therapy in this patient population. Therefore, the aim of our study was to investigate possible alterations in lung function of patients with lung cancer that underwent chemoradiation associating the three most common platinum combinations such as vinorelbine (VN), etoposide (EP), and gemcitabine (GEM).
Methods
Patients
Inclusion criteria were NSCLC stage IIIB or IV necessitating thoracic irradiation, SCLC with endothoracic disease, Eastern Cooperative Oncology Group (ECOG) PS 0 or 1, adequate imaging, not severe comorbidity, adequate blood tests. Any progression or PS deterioration or serious adverse event including grade III/IV toxicity conducted to patient’s protocol exit. Ethical approval was obtained from the Internal Review Board of the University Hospital-Medical School Democritus University of Thrace (IRB No. 4360/01-11-2007). All patients signed an informed consent prior to the study inclusion. Twenty patients with histologically or cytologically proven lung cancer were included in this study within a two-year period. Fifteen were finally evaluable because 5 of them progressed during chemotherapy becoming stage IV and radiation therapy was not further indicated.
Treatment schedule
All patients were treated with chemotherapy prior to radiation therapy. They were assigned according to their histological type to one of the three platinum-based chemotherapy groups. Treatment schedules consisted of cisplatin-VN (6 patients), cisplatin-GEM (4 patients) or cisplatin-EP (5 patients). The first group received 30 mg/m2 of VN, administered on days 1 and 8. The second group received 1,000 mg/m2 of GEM on days 1 and 8 and the third group 150 mg/m2 of EP on days 1 to 3. All three compounds were associated to cisplatin on day 1 at a dose of 100 mg/m2. Treatment was repeated in each group every 3 weeks for a total of six planned courses. All evaluable patients had to fully comply with treatment.
Radiotherapy (RT) was delivered to the affected lung and mediastinal nodes. All patients were treated with 18-MV photons and three-dimension conformal RT, which spares healthy tissue as previously described (10). Nine fractions of 3.5 Gy were applied, delivering a median biological (for α/β=4 Gy dose) of 48 Gy to the mediastinum and the tumour mass, followed by five additional fractions of 3.5 Gy that increased the total biological dose to >70 Gy to the gross tumor area.
PFTs
PFTs were first performed before the beginning of the treatment (T0). The second measurement was carried out after the six planned chemotherapy courses, just before radiation therapy (T1) and the third, one month after the end of radiation therapy (T2). PFTs were measured according to the ATS/ERS guidelines (11-13). They consisted in forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), total lung capacity (TLC), diffusing capacity for carbon monoxide (DLCO) and carbon monoxide transfer coefficient (Kco). Lung volumes performed using body-box plethysmography by the helium dilution method. DLCO was measured by the single-breath breath-holding technique. DLCO was adjusted for actual haemoglobin concentration and for alveolar volume (VA) and the carbon monoxide transfer coefficient (Kco) was calculated as the ratio of DLco/VA. Results of the tests were expressed as the percentage of the predicted value for each patient. Changes in the PFTs values after chemotherapy and radiation therapy were expressed as the difference between pre and post treatment values relative to the pretreatment values. PFTs were performed with a Jaeger Masterlab System (E. Jaeger GmbH, Wuerzburg, Germany).
Statistical analysis
All data are expressed as mean values ± standard deviation (±SD). Comparison of means between groups was performed with the Student’s t-test. PFTs data analysis over time was assessed by the analysis of variances (ANOVA). The effect of treatment on the PFTs was assessed using the Friedman test as data were abnormally distributed, due to the small subgroups. Statistical significance of all tests was set at a P value of less than 0.05. A statistical software package (StatViewTM version 4.5, Abacus Concepts Inc., Berkeley, CA, USA) was used for data analysis.
Results
Patients’ characteristics are shown in Table 1. All patients but one were males and smokers (14/15, 93.3%) with a median of 76.4±29.4 pack-years. Mean patients’ age was 58±9.4 years, ranged from 42 to 75 years. Ten patients (66.6%) had a PS =0 and five (33.3%) had PS =1. NSCLC was the histological type in 10 patients and SCLC in 5 (Table 1). No significant difference was noted in the patients’ age (overall P=0.60), nor in the smoking status (overall P=0.34) between the three treated groups (Table 1). Also no significant differences were observed between the PFTs baseline values for the three chemotherapeutic regimens (Table 2).
Full table

Full table
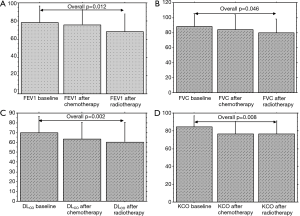
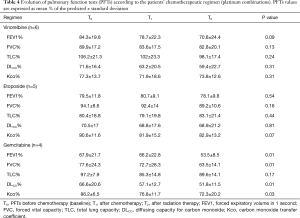
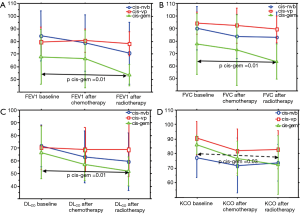
PFTs of all patients according to the time point are shown in Table 3. Overall significant changes (Figure 1A,B,C,D) were observed from the baseline (To) mean values in FEV1 (P=0.012), in FVC (P=0.046), DLCO (P=0.002) and KCO (P=0.008). When adjusted to the treatment group, this decline of PFTs was only observed with the combination of cisplatin and GEM (Table 4 and Figure 2).

Full table
Full table
Discussion
Our patients were treated by the association of chemotherapy and radiation therapy as several studies demonstrated that this combination improves the outcome of patients with locally advanced lung cancer (9). However, the combination of chemotherapy and RT can increase the extent of pulmonary injury and the risk for radiation pneumonitis (14-17). Radiation therapy is associated with significant alterations in DLCO and KCO (18-20), as was observed in our study. The decrease in DLCO is a result of limited gas exchange reserve, caused by the potential toxicity of treatment. This decrease, may reflect interstitial and parenchymal damage (21) leading to impairment in diffusion capacity of the alveolar membrane (22). Radiation results in the damage of endothelial, epithelial (type II pneumocytes) cells, by apoptosis and stimulation of stress response genes progressing to an acute exudative inflammation process, which occurs usually 1–6 months after completion of treatment (4,5). Also, early histological lesions with various grades of interstitial and parenchymal involvement were observed after both chemotherapy and radiation therapy (10).
In our study, overall, a progressive decline of FEV1 and FVC was observed after chemoradiation. In other studies early increases, yet not statistically significant, were observed in FEV1 and FVC after induction chemotherapy (18,19). The same observation has been noted in patients treated by chemotherapy alone (23,24). This observation might be due to the relief of obstruction in centrally located tumors. Yet, after long-term surveillance, a significant reduction in pulmonary function was apparent between 3 (25) and 6 (26) months, as in our study, with no recovery until 36 months (25) after radiation therapy. In a retrospective analysis of 60 patients treated in a concurrent chemoradiation schedule (27), both FEV1 and FVC were significantly decreased at 6 months and remained low at 12 (27). This decrease was related to the intensity of radiation therapy. Yet, 37% of these patients developed grade 3 or 4 radiation pneumonitis (27).
The drop in PFTs in our study was significantly greater in the group of patients treated with cisplatin-GEM. Patients of this group had the worst, although not significant, pulmonary function before chemoradiation, compared with the other two combinations. It has been suggested that FEV1 before treatment is important in lung damage development, and this risk increases in patients with low FEV1 (28). However, GEM is a well-known agent with lung toxicity, used as a single agent or in combination with other chemotherapeutic drugs (22,29). GEM may cause diffuse ground-glass changes accompanied by thickened septal lines, interstitial or diffuse alveolar infiltrates, which may sometimes be associated with acute respiratory distress syndrome and, rarely, death (30). This drug appears to have a direct toxic effect on the endothelial cells of the pulmonary capillaries, which causes a capillary leak syndrome (22,29).
The current study has several limitations. First, the number of patients analyzed is relatively small, due to recruitment difficulties. Indeed, in such patient population, the disease progression as well as the low PS is factors of poor accrual. Second, there is obviously a significant difficulty to assess the long-term impact of the therapy on lung function because of the low survival rates in this patient population. Third, the lack of correlation of the PFT data with the response to treatment. Actually, no data exist about the degree of tumour response in regards to the treatment consequences on the lung environment such, as pneumonitis or fibrosis, after chemoradiation, based on radiological or histological findings.
To conclude, our prospective study showed changes in some of the lung function parameters assessed in patients with locally advanced lung cancer after chemoradiation. However, as our population was limited in number, large prospective studies are needed to further assess the effect of chemoradiation on the lung.
Acknowledgements
This research was supported with a grant from “Fondation Lancardis”, Martigny, Switzerland.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Internal Review Board of the University Hospital-Medical School Democritus University of Thrace (No. 4360/01-11-2007) and written informed consent was obtained from all patients.
References
- Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol 2013;30:93-8. [Crossref] [PubMed]
- Cooper JA Jr, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1: Cytotoxic drugs. Am Rev Respir Dis 1986;133:321-40. [PubMed]
- Kreisman H, Wolkove N. Pulmonary toxicity of antineoplastic therapy. Semin Oncol 1992;19:508-20. [PubMed]
- Movsas B, Raffin TA, Epstein AH, et al. Pulmonary radiation injury. Chest 1997;111:1061-76. [Crossref] [PubMed]
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281-93. [Crossref] [PubMed]
- Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5-24. [Crossref] [PubMed]
- Margaritora S, Cesario A, Cusumano G, et al. Is pulmonary function damaged by neoadjuvant lung cancer therapy? A comprehensive serial time-trend analysis of pulmonary function after induction radiochemotherapy plus surgery. J Thorac Cardiovasc Surg 2010;139:1457-63. [Crossref] [PubMed]
- Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 2005;23:5910-7. [Crossref] [PubMed]
- De Ruysscher D, Lueza B, Le Pechoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol 2016;27:1818-28. [Crossref] [PubMed]
- Karpathiou G, Giatromanolaki A, Koukourakis MI, et al. Histological changes after radiation therapy in patients with lung cancer: a prospective study. Anticancer Res 2014;34:3119-24. [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005;26:511-22. [Crossref] [PubMed]
- Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720-35. [Crossref] [PubMed]
- Theuws JC, Muller SH, Seppenwoolde Y, et al. Effect of radiotherapy and chemotherapy on pulmonary function after treatment for breast cancer and lymphoma: A follow-up study. J Clin Oncol 1999;17:3091-100. [Crossref] [PubMed]
- Lind PA, Rosfors S, Wennberg B, et al. Pulmonary function following adjuvant chemotherapy and radiotherapy for breast cancer and the issue of three-dimensional treatment planning. Radiother Oncol 1998;49:245-54. [Crossref] [PubMed]
- Lingos TI, Recht A, Vicini F, et al. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys 1991;21:355-60. [Crossref] [PubMed]
- McDonald S, Rubin P, Phillips TL, et al. Injury to the lung from cancer therapy: clinical syndromes, measurable endpoints, and potential scoring systems. Int J Radiat Oncol Biol Phys 1995;31:1187-203. [Crossref] [PubMed]
- Takeda S, Funakoshi Y, Kadota Y, et al. Fall in diffusing capacity associated with induction therapy for lung cancer: a predictor of postoperative complication? Ann Thorac Surg 2006;82:232-6. [Crossref] [PubMed]
- Cerfolio RJ, Talati A, Bryant AS. Changes in pulmonary function tests after neoadjuvant therapy predict postoperative complications. Ann Thorac Surg 2009;88:930-5; discussion 935-6. [Crossref] [PubMed]
- Gopal R, Starkschall G, Tucker SL, et al. Effects of radiotherapy and chemotherapy on lung function in patients with non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:114-20. [Crossref] [PubMed]
- Froudarakis M, Hatzimichael E, Kyriazopoulou L, et al. Revisiting bleomycin from pathophysiology to safe clinical use. Crit Rev Oncol Hematol 2013;87:90-100. [Crossref] [PubMed]
- Briasoulis E, Pavlidis N. Noncardiogenic pulmonary edema: an unusual and serious complication of anticancer therapy. Oncologist 2001;6:153-61. [Crossref] [PubMed]
- Dimopoulou I, Galani H, Dafni U, et al. A prospective study of pulmonary function in patients treated with paclitaxel and carboplatin. Cancer 2002;94:452-8. [Crossref] [PubMed]
- Dimopoulou I, Efstathiou E, Samakovli A, et al. A prospective study on lung toxicity in patients treated with gemcitabine and carboplatin: clinical, radiological and functional assessment. Ann Oncol 2004;15:1250-5. [Crossref] [PubMed]
- Borst GR, De Jaeger K, Belderbos JS, et al. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys 2005;62:639-44. [Crossref] [PubMed]
- Miller KL, Zhou SM, Barrier RC Jr, et al. Long-term changes in pulmonary function tests after definitive radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2003;56:611-5. [Crossref] [PubMed]
- Park YH, Kim JS. Predictors of radiation pneumonitis and pulmonary function changes after concurrent chemoradiotherapy of non-small cell lung cancer. Radiat Oncol J 2013;31:34-40. [Crossref] [PubMed]
- Robnett TJ, Machtay M, Vines EF, et al. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2000;48:89-94. [Crossref] [PubMed]
- Briasoulis E, Froudarakis M, Milionis HJ, et al. Chemotherapy-induced noncardiogenic pulmonary edema related to gemcitabine plus docetaxel combination with granulocyte colony-stimulating factor support. Respiration 2000;67:680-3. [Crossref] [PubMed]
- Belknap SM, Kuzel TM, Yarnold PR, et al. Clinical features and correlates of gemcitabine-associated lung injury: findings from the RADAR project. Cancer 2006;106:2051-7. [Crossref] [PubMed]


