Role of p16 deletion and BAP1 loss in the diagnosis of malignant mesothelioma
Introduction
Malignant mesothelioma (MM) is a rare but highly aggressive neoplasm. It has a poor prognosis and a median survival time of 20 months after diagnosis (1). Tumor development is associated with exposure to several known carcinogens such as asbestos fiber, rhesus virus 40, and radiation, of which asbestos exposure is the most important risk factor (2). The early clinical symptoms of MM lack specificity and are often characterized by large amounts of pleural effusion, which make clinical and imaging diagnoses difficult. Mesothelioma often goes undiagnosed until the late stages of the disease, at which time no marked effects can be achieved, regardless of whether the patient and medical team select surgery or radiotherapy and chemotherapy.
Differential diagnosis of MM from reactive mesothelial hyperplasia (RMH) or metastatic carcinoma is crucial to patient care and prognosis, but distinguishing these specific conditions from each other can be very difficult. RMH is a benign condition, but it often mimics the features of neoplasm including high cellularity, the presence of numerous mitotic figures and cytologic atypia, the formation of papillary groups, the presence of necrosis, and entrapment of mesothelial cells within fibrosis, mimicking invasion (3). This makes MM and RMH appear very similar in histological appearance. Immunohistochemistry (IHC) for calretinin, podoplanin, cytokeratin 5/6, and Wilms’ tumor-1 proteins are commonly used to diagnosis MM, with a sensitivity of greater than 90%, 90–100%, 75–100%, and 70–95%, respectively (3). Although these immunohistochemical markers have high sensitivity for MM, they are also positively expressed in some RMH cases (4). Similarly, histopathologic differentiation between MM and lung adenocarcinoma is often challenging. Epithelial MM is a tumor with a small tube, acinar, flaky atypical growth pattern formed by epithelial mesothelial cells, similar to the histological structure of lung adenocarcinoma. Distinguishing MM from lung adenocarcinomas can be difficult, even when multiple immunohistochemical stains are deployed. Sometimes MM expresses “carcinoma” markers such as epithelial membrane antigen (5), and lung adenocarcinomas do not always express thyroid transcription factor 1 or Napsin A, so their sensitivity is not more than 80% (6). For this reason, it is important to identify more specific markers that facilitate the early and accurate diagnosis of MM.
Recently two new markers that appeared to be useful for distinguishing benign from MM were discovered, namely p16 [cyclin-dependent kinase inhibitor 2A (CDKN2A)], a tumor suppressor gene, and BRCA1-associated protein 1 (BAP1). Deletion of p16 has proven to be a reliable way of differentiating benign from MM proliferation, which is one of the most common cytogenetic abnormalities in MM and can be detected by fluorescence in situ hybridization (FISH). P16 deletion has been found in 47.4–81.3% of MM cases, but no cases of RMH have been reported to harbor p16 deletion (4,5,7,8). However, even at a specificity of 100%, some MM cases do not show p16 deletion. Thus, another useful marker, BAP1 was identified. Previous studies have shown that BAP1 loss can occur as a result of gene deletion, point mutations, or other indirect mechanisms and BAP1 loss can be assessed by IHC (9,10). Loss of BAP1 staining has been observed in many tumors such as epithelioid atypical Spitz tumors, melanoma, renal cell carcinoma, and MM (11). The relationship between BAP1 loss and MM is significant. BAP1 loss has been reported in 15–67.5% of MM cases, with a specificity of 100% for differentiating between MM and RMH (4,7,12-15). Thus together, p16 and BAP1 are reliable markers for differentiating MM from RMH.
BAP1 has also been used to identify MM and lung adenocarcinoma. Although the correlation between BAP1 loss and lung cancer is not completely understood, it is known that loss of BAP1 expression is rare in lung adenocarcinoma (16,17). Thus, it is reasonable to assume that loss of BAP1 in a thoracic malignancy would provide strong support for the diagnosis of MM.
The goal of this study was to establish the value of p16 deletion detected by FISH and BAP1 loss detected by IHC for MM diagnosis, and to determine the value of p16 deletion and BAP1 loss in distinguishing MM from RMH and lung adenocarcinoma.
Methods
Patients
We retrospectively reviewed 78 cases from 2011 to 2017 including 35 cases of MM, 9 cases of RMH, and 33 cases of lung adenocarcinoma. All patients were diagnosed at the First Affiliated Hospital of Guangzhou Medical University (Guangdong, China). Tissue paraffin block from all cases were preserved. In total, 26 MM cases and 5 RMH cases were biopsy specimens; and 9 MM cases, 4 RMH cases, and all lung adenocarcinoma cases were resection specimens. Clinical data collected included age, sex, and smoking history.
FISH
Commercially available SpectrumOrange-labeled locus-specific p16 (9p21) probe and SpectrumGreen-labeled chromosome 9 centromeric probe (Vysis LSI CDKN2A SpectrumOrange/CEP9 SpectrumGreen Probe; Abbott Laboratories, Chicago, IL, USA) were used for the dual-color FISH studies, which were performed on formalin-fixed, paraffin-embedded histologic sections of 3 µm in thickness. The paraffin sections were deparaffinized in xylene, followed by rehydration and pretreatment in deionized water at 100 °C for 15 min. After immersion in 2× saline sodium citrate (SSC; Sigma, St. Louis, MO, USA) for 5 min, slides were digested by proteinase K (20 mg/L in 2× SSC; Sigma) at 37 °C for 5 min in a humidified chamber, washed in 2× SSC again for 5 min, and air dried at room temperature. Slides were dehydrated and co-denatured with the probes for 5 min at 80 °C, hybridized at 42 °C for 18 h in the ThermoBrite unit (Abbott Japan, Tokyo, Japan), washed with 2× SSC/0.1% NP40 at 46 °C for 10 min, and counterstained with DAPI/antifade (Abbott, Japan). The slides were scored on a cell-by-cell basis using an Olympus microscope (Olympus, Tokyo, Japan) with a single band pass filter for DAPI, SpectrumGreen, and SpectrumOrange. A hematoxylin and eosin-stained section was used to verify the presence of the tumor. At least 100 cells were scored for each case.
IHC
IHC was performed on formalin-fixed, paraffin-embedded tissue sections of 4 µm in thickness. Tissue sections were baked at 65 °C overnight and then an automatic immunohistochemical instrument (LEICA, Wetzlar, Germany) and mouse monoclonal anti-human BAP1 antibody (clone C-4, 1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for IHC. After automated immunohistochemical treatment, the sample was washed with anhydrous alcohol and the slide was allowed to dry. An automatic sealing machine (LEICA) was used to seal the slice. All sections were observed under an Olympus microscope (Olympus). The absence of nuclear immunoreactivity in the presence of preserved immunoreactivity in the lymphocytes and fibroblasts of tumors was indicative of BAP1 loss. The number of tumor cells with BAP1 loss was determined as the percentage of all tumor cells.
Cut-off values for normal p16 FISH signaling
Nuclear signal deletion was divided into two main types: homozygous deletion and hemizygous deletion. P16 homozygous deletion was defined as loss of both p16/CDKN2A signals with at least one centromere enumeration probe 9 (CEP-9) signal (0SpO/1-2SpG), and hemizygous deletion was defined as the presence of only one p16 signal in cells with two CEP-9 signals (1SpO/2SpG). Because sectioning of paraffin-embedded blocks creates nuclei truncation and some deletions can be caused by signal loss, a cut-off value for normal p16 FISH signaling was established. We used methods described in previous works to select the cut-off value for normal p16 FISH signaling, and at least 100 cells were scored for each case (8). In this study, cut-off levels were calculated as the mean percentage + four standard deviations of nuclei of RMH. We calculated the frequency of p16 deletion in the nine cases of RMH. Here, 1–8% (mean, 3.2%) of the cells had homozygous deletions, and 2–11% (mean, 4.8%) had hemizygous deletions. In this work, 5–16% (mean, 8.3%) of each nucleus showed homozygous or hemizygous deletions. P16 homozygous deletion was defined as more than 12.1% nuclei exhibiting a 0SpO/1–2SpG pattern, hemizygous deletion was defined as more than 15.7% nuclei exhibiting a 1SpO/2SpG pattern, and homozygous or hemizygous deletion was defined as more than 24.5% nuclei exhibiting a 0SpO/1–2SpG or 1SpO/2SpG pattern. The cut-off value of normal p16 FISH signaling in lung tissue was shown in our previous study (18). It was set according to the nuclear signal of p16 in normal lung tissue or lung tissue with inflammation and benign alveolar epithelial hyperplasia. P16 homozygous deletion was defined as more than 13.2% of nuclei showing a 0SpO/1–2SpG pattern, p16 hemizygous deletion was defined as more than 34.2% of nuclei showing a 1SpO/2SpG pattern, and homozygous/hemizygous deletion was defined as more than 41.8% of nuclei showing a 0SpO/1–2SpG or 1SpO/2SpG pattern.
BAP1 IHC cut-off values
Although BAP1 IHC is unambiguously and homogeneously expressed in the nuclei of most tumor cells, heterogenous BAP1 expression has also been observed in several cases. We used previously published methods to establish cut-off values for normal BAP1 expression, as determined by IHC; at least 500 cells were scored for each case (7). In this work, we calculated the proportion of BAP1-IHC-positive cells among the tumor cells, and receiver operating curve analysis was used to establish the cut-off value for the BAP1 IHC assay. Fewer than 19.7% of cells expressed BAP1, indicating some BAP1 loss. According to this cut-off value, the sensitivity and specificity were 67.5% and 100%, respectively, for MM diagnosis. We also used this cut-off value to assess the error caused by heterogenous BAP1 expression.
Statistical analysis
Statistical analysis was performed using SPSS Statistics for Windows, Version 16.0, and the χ2 test was used to compare the clinical data. BAP1 and p16 were used to correlate MM with relevant matching data. P<0.05 was considered statistically significant.
Results
Clinical features
The clinical features of the 35 patients with MM, 9 patients with RMH, and 33 patients with lung adenocarcinoma are summarized in Table 1.
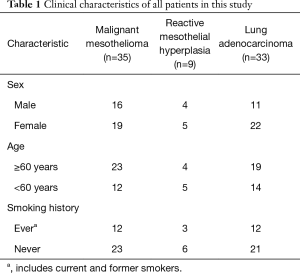
Full table
p16 FISH
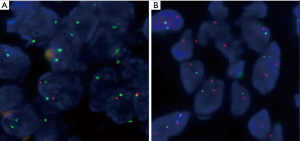
FISH analysis was successful in 32 of the 35 MM cases. The remaining three cases were removed because they had fewer than 100 tumor cells, including two cases of biopsies and one case of resection. Of the remaining 32 patients who had undergone p16 FISH, 62.5% (20/32) showed homozygous deletion, 9.4% (3/32) showed hemizygous deletion, and 28.1% (9/32) showed no deletions. A total of 9 patients with RMH and 33 with lung adenocarcinoma successfully underwent FISH. None of the RMH cases had p16 deletions. Here, 45.5% (15/33) of lung adenocarcinoma patients were found to have p16 deletions, all of which were homozygous deletion (Figure 1). According to the results shown in Table 2, the difference between MM and RMH had obvious statistical significance (P<0.01). Table 3 shows the results of p16 FISH in the MM cases. There was no correlation between p16 FISH expression and the age or sex of MM patients, but their smoking history correlated with p16 expression, as detected by FISH (P<0.05).
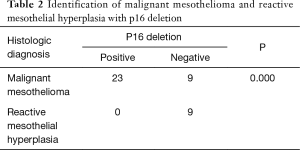
Full table
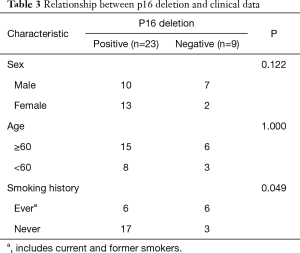
Full table
BAP1 IHC
The BAP1 IHC assay was successfully performed for the 35 MM cases, 9 RMH cases, and 33 lung adenocarcinoma cases (Figure 2). Of the MM cases, 37.1% (13/35) showed loss of BAP1 expression, of which 7 of the 13 cases showed p16 homozygous deletion, 1 showed hemizygous deletion, and 2 had fewer than 100 tumor cells. All of the RMH and lung adenocarcinoma cases had BAP1 expression. Tables 4,5 showed that the difference between MM and RMH or lung adenocarcinoma was statistically significant (P<0.05). According to the results of the Fisher’s exact test, there was no correlation between BAP1 expression and age, sex, or smoking history in patients with MM (Table 6).
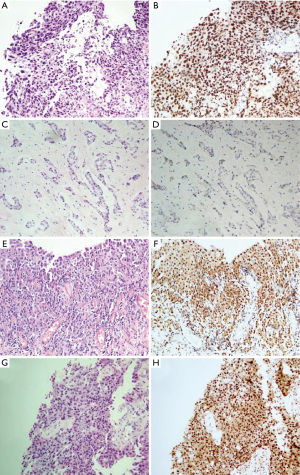
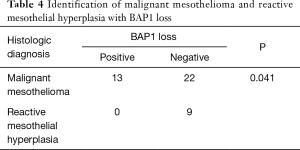
Full table

Full table
Full table
Relationship between p16 FISH and BAP1 IHC for MM diagnosis
Next, we investigated the correlation between p16 deletion and BAP1 loss. Table 7 shows the results of p16 FISH and BAP1 IHC for MM. Analysis of the matched data showed that there was no correlation between the results of the two methods for diagnosing MM (P>0.05). However, when the methods were used in combination, they showed greater sensitivity (from 71.9% to 80%) than BAP1 IHC or p16 FISH alone.
Full table
Discussion
The results of this study demonstrate that p16 deletion and BAP1 loss have excellent clinical utility for the diagnosis of MM. In addition, they can be used to differentiate benign from MM proliferation, and BAP1 loss can be used to distinguish MM from lung adenocarcinoma.
P16 is a tumor suppressor gene located on human chromosome 9 short arm 2 zone 1 band (9p21) (19), and is directly involved in regulation of the cell cycle and the negative regulation of cell proliferation and division. Abnormal p16 expression is closely related to the development of various tumors. Many studies have shown that p16 deletion is useful for differentiating between MM and RMH, but to date, there have been no published reports on the role of p16 deletion in RMH (20-22). In this study, the sensitivity of p16 FISH was 71.9% (23/32) for MM diagnosis and none of the RMH cases showed p16 deletion, consistent with previous findings. Although this marker has relatively high sensitivity, some patients with MM do not show p16 deletion, so another useful marker, BAP1, was evaluated in this study.
BAP1 is located on chromosome 3 (3p21.1). It is a nuclear ubiquitin hydrolase that regulates the cell cycle, cellular differentiation, transcription, and DNA repair and functions as a classical tumor suppressor (23). Several studies have revealed that BAP1 protein expression is often lost in MM (7,12,24). In this study, the sensitivity of BAP1 IHC was 37.1% (13/35) for MM diagnosis, and the specificity of distinguishing MM from RMH was 100%. The sensitivity was not as high as that described by Hida et al., who cited 67.5% using BAP1 IHC for MM diagnosis, although the same cut-off values were used. The difference in results may be due to the smaller sample sizes used in this study.
The problem with both p16 FISH and BAP1 IHC is that each has limited sensitivity. However, their combined use was expected to increase the sensitivity, resulting in greater confidence in the accuracy of MM diagnosis. Based on the estimated probabilities for the results of each assay in our study, combined testing produced a significant improvement (from 71.9% to 80%) compared to p16 FISH testing alone, in accordance with previous studies showing that the combined use of BAP1 IHC and p16 FISH assays increased the sensitivity (7,15,25).
Differentiating among MM, non-small cell lung cancer, and especially adenocarcinoma, once they metastasize to the pleura or peritoneum can be difficult. It has also difficult to find novel diagnostic markers that can be used to distinguish between MM and lung adenocarcinoma. According to previous studies, p16 deletion occurs in 29–59% of primary lung adenocarcinomas (26-29); 45.5% (15/33) of adenocarcinomas showed p16 deletion in our study, which made it impossible to use this marker to differentiate MM from lung adenocarcinoma. None of the patients (0%, 0/33) with adenocarcinoma showed BAP1 loss, confirming that BAP1 loss is rare in lung adenocarcinoma (16,17,30).
BAP1 can be used to distinguish MM from lung adenocarcinoma. Some studies have also reported that Ber-EP4 and MOC-31 immunostaining may be useful for this purpose as well (31-34). Ber-EP4 is a monoclonal antibody distinct from keratin or other common epithelial-associated antigens, which reacts with most epithelial cells and neoplasms (35). MOC31 is a cell surface glycoprotein of unknown function, and the pattern of reaction is plasmalemmal. MOC31 is expressed in most normal and malignant epithelial cells (36). Although multiple studies have shown that Ber-EP4 and MOC31 are positive markers for lung adenocarcinoma and negative markers for MM, they are not expressed in every case of lung adenocarcinoma (30,37) and they occasionally stain a small fraction of MM cells (38-40). Therefore, although the diagnostic value of BAP1 for this differential diagnosis is limited due to low sensitivity of BAP1 loss in mesothelioma, it can also be used as a supplementary marker in discriminating MM from lung adenocarcinoma. Thus, IHC for BAP1 can be added to the clinical diagnostic panel used in the context of the differential diagnosis of MM from lung adenocarcinomas.
The results of this study showed that p16 deletion showed higher sensitivity for MM than BAP1 loss. P16 deletion could only be detected by FISH and not by IHC. Loss of p16 expression occurs not only through p16 deletion but also through p16 methylation or other genetic abnormalities. However, in contrast to p16 deletion, which can only be detected by FISH, BAP1 loss can be conveniently assessed by IHC. FISH is more expensive, more labor intensive, and less likely to be available in clinical laboratories than IHC. Thus, although it has low sensitivity, BAP1 IHC may still be a good starting point for testing because it is faster and cheaper than p16 FISH, and when used in combination with FISH, the accuracy of MM diagnosis will increase.
Conclusions
P16 and BAP1 both showed independent diagnostic value for the diagnosis of MM, and the combination of p16 deletion detected by FISH and BAP1 loss detected by IHC detected MM with greater sensitivity greater than each method used alone. BAP1 is also a good marker for distinguishing MM from lung adenocarcinoma. Future studies should include greater sample sizes, and the relationship between p16 and BAP1 expression and the prognosis of MM should be separately analyzed.
Acknowledgements
We would like to thank LetPub (www.LetPub.com) for providing linguistic assistance during the preparation of this manuscript.
Funding: This work was supported by the Research Fund for Higher Education of Guangzhou (No. 1201620051 to J Jiang) and the National Natural Science Foundation of China (No. 81272901 to J Jiang).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pasello G, Favaretto A. Molecular targets in malignant pleural mesothelioma treatment. Current Drug Targets 2009;10:1235-44. [Crossref] [PubMed]
- Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res 2012;18:598-604. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647. [Crossref] [PubMed]
- Hwang HC, Pyott S, Rodriguez S, et al. BAP1 Immunohistochemistry and p16 FISH in the Diagnosis of Sarcomatous and Desmoplastic Mesotheliomas. Am J Surg Pathol 2016;40:714. [Crossref] [PubMed]
- Ito T, Hamasaki M, Matsumoto S, et al. p16/CDKN2A FISH in Differentiation of Diffuse Malignant Peritoneal Mesothelioma From Mesothelial Hyperplasia and Epithelial Ovarian Cancer. Am J Clin Pathol 2015;143:830-8. [Crossref] [PubMed]
- Galateau-Salle F, Churg A, Roggli V, et al. The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification. J Thorac Oncol 2016;11:142-54. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. BAP1 immunohistochemistry and p16 FISH results in combination provide higher confidence in malignant pleural mesothelioma diagnosis: ROC analysis of the two tests. Pathol Int 2016;66:563-70. [Crossref] [PubMed]
- Wu D, Hiroshima K, Matsumoto S, et al. Diagnostic usefulness of p16/CDKN2A FISH in distinguishing between sarcomatoid mesothelioma and fibrous pleuritis. Am J Clin Pathol 2013;139:39-46. [Crossref] [PubMed]
- Zauderer MG, Bott M, McMillan R, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol 2013;8:1430-3. [Crossref] [PubMed]
- McGregor SM, Dunning R, Hyjek E, et al. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol 2015;46:1670-8. [Crossref] [PubMed]
- Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology 2013;45:116-26. [Crossref] [PubMed]
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72. [Crossref] [PubMed]
- McGregor SM, McElherne J, Minor A, et al. BAP1 immunohistochemistry has limited prognostic utility as a complement of CDKN2A (p16) fluorescence in situ hybridization in malignant pleural mesothelioma. Hum Pathol 2017;60:86-94. [Crossref] [PubMed]
- Walts AE, Hiroshima K, McGregor SM, et al. BAP1 Immunostain and CDKN2A (p16) FISH Analysis: Clinical Applicability for the Diagnosis of Malignant Mesothelioma in Effusions. Diagn Cytopathol 2016;44:599-606. [Crossref] [PubMed]
- Hwang HC, Sheffield BS, Rodriguez S, et al. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the Diagnosis of Malignant Mesothelioma in Effusion Cytology Specimens. Am J Surg Pathol 2016;40:120. [Crossref] [PubMed]
- Andrici J, Parkhill TR, Jung J, et al. Loss of expression of BAP1 is very rare in non-small cell lung carcinoma. Pathology 2016;48:336-40. [Crossref] [PubMed]
- Owen D, Sheffield BS, Ionescu D, et al. Loss of BRCA1-associated protein 1 (BAP1) expression is rare in non-small cell lung cancer. Hum Pathol 2017;60:82-5. [Crossref] [PubMed]
- Jiang J, Gu Y, Liu J, et al. Coexistence of p16/CDKN2A homozygous deletions and activating EGFR mutations in lung adenocarcinoma patients signifies a poor response to EGFR-TKIs. Lung Cancer 2016;102:101-7. [Crossref] [PubMed]
- Kamb A, Gruis NA, Weaverfeldhaus J, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994;264:436-40. [Crossref] [PubMed]
- Churg A, Sheffield BS, Galateau-Salle F. New Markers for Separating Benign From Malignant Mesothelial Proliferations: Are We There Yet? Arch Pathol Lab Med 2016;140:318-21. [Crossref] [PubMed]
- Monaco SE, Shuai Y, Bansal M, et al. The diagnostic utility of p16 FISH and GLUT-1 immunohistochemical analysis in mesothelial proliferations. Am J Clin Pathol 2011;135:619-27. [Crossref] [PubMed]
- Chung CT, Santos Gda C, Hwang DM, et al. FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J Clin Pathol 2010;63:630-4. [Crossref] [PubMed]
- Ventii KH, Devi NS, Friedrich KL, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008;68:6953. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977-82. [Crossref] [PubMed]
- Tam KW, Zhang W, Soh J, et al. CDKN2A/p16 inactivation mechanisms and their relationship to smoke exposure and molecular features in non-small-cell lung cancer. J Thorac Oncol 2013;8:1378-88. [Crossref] [PubMed]
- Iwakawa R, Kohno T, Anami Y, et al. Association of p16 homozygous deletions with clinicopathologic characteristics and EGFR/KRAS/p53 mutations in lung adenocarcinoma. Clin Cancer Res 2008;14:3746-53. [Crossref] [PubMed]
- Kraunz KS, Nelson HH, Lemos M, et al. Homozygous deletion of p16INK4a and tobacco carcinogen exposure in non-small cell lung cancer. Int J Cancer 2006;118:1364-9. [Crossref] [PubMed]
- Hamada K, Kohno T, Kawanishi M, et al. Association of CDKN2A (p16)/CDKN2B (p15) alterations and homozygous chromosome arm 9p deletions in human lung carcinoma. Genes Chromosomes Cancer 1998;22:232. [Crossref] [PubMed]
- Jaouen A, Thivolet-Bejui F, Chalabreysse L, et al. Apport de l’expression protéique de BRCA1 associated protein 1 (BAP1) dans le diagnostic des mésothéliomes malins diffus pleuraux: une analyse cytologique et histologique comparative sur une série de 50 patients. Annales De Pathologie 2016;36:111-9. [Crossref] [PubMed]
- Sheibani K, Shin SS, Kezirian J, et al. Ber-EP4 antibody as a discriminant in the differential diagnosis of malignant mesothelioma versus adenocarcinoma. Am J Surg Pathol 1991;15:779-84. [Crossref] [PubMed]
- Oates J, Edwards C. HBME-1, MOC-31, WT1 and calretinin: an assessment of recently described markers for mesothelioma and adenocarcinoma. Histopathology 2000;36:341. [Crossref] [PubMed]
- Betta PG, Magnani C, Bensi T, et al. Immunohistochemistry and molecular diagnostics of pleural malignant mesothelioma. Arch Pathol Lab Med 2012;136:253. [Crossref] [PubMed]
- Ordóñez NG. Value of the Ber-EP4 antibody in differentiating epithelial pleural mesothelioma from adenocarcinoma. The M.D. Anderson experience and a critical review of the literature. Am J Clin Pathol 1998;109:85-9. [Crossref] [PubMed]
- Latza U, Niedobitek G, Schwarting R, et al. Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol 1990;43:213-9. [Crossref] [PubMed]
- Chang K, Pai LH, Pass H, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol 1992;16:259-68. [Crossref] [PubMed]
- Carella R, Deleonardi G, D'Errico A, et al. Immunohistochemical panels for differentiating epithelial malignant mesothelioma from lung adenocarcinoma: a study with logistic regression analysis. Am J Surg Pathol 2001;25:43-50. [Crossref] [PubMed]
- Pu RT, Pang Y, Michael CW. Utility of WT-1, p63, MOC31, mesothelin, and cytokeratin (K903 and CK5/6) immunostains in differentiating adenocarcinoma, squamous cell carcinoma, and malignant mesothelioma in effusions. Diagn Cytopathol 2008;36:20-5. [Crossref] [PubMed]
- Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031-51. [Crossref] [PubMed]
- Carbone M, Guo Z, Mao W. Improving the Accuracy of Mesothelioma Diagnosis in China. J Thorac Oncol 2017;12. [Crossref] [PubMed]


