Association of eosinophil-to-monocyte ratio with 1-month and long-term all-cause mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention
Introduction
ST-segment elevation myocardial infarction (STEMI) is a severe type of coronary artery disease (CAD) with high morbidity and mortality (1). Inflammatory and immunological responses play an irreplaceable role in the pathogenesis of STEMI (2,3). Leukocytes including neutrophils (4), monocytes (5), and lymphocytes (6) are indispensable inflammatory cells and are associated with atherosclerosis development and progression, plaque rupture, vascular dysfunction and left ventricular remodeling in STEMI patients (7,8). Recently, neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) were found to be independent predictive factors of the severity and clinical prognosis of STEMI (9-12). Nevertheless, the association of these individual indexes with cardiovascular diseases has been inconsistent because of their weak specificity (13). There are reports that eosinophils were also inflammatory cells and were able to regulate the inflammatory progress (14). We are not sure whether eosinophil-to-monocyte ratio (EMR) has the similar predictive value as NLR and LMR. This present study assessed the relationship between EMR and one-month and long-term all-cause mortality in STEMI patients undergoing primary percutaneous coronary intervention (P-PCI).
Methods
Study population
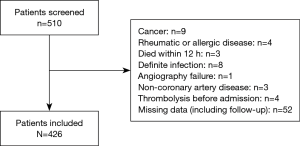
As shown in Figure 1, a total of 510 consecutive patients with STEMI who received P-PCI between September 2015 and October 2017 at our center were retrospectively recorded. 84 were excluded because of the following criteria: presence of cancer (n=9), or rheumatic or allergic disease (n=4); death within 12 h (n=3); confirmed infection (n=8); unsuccessful angiography (n=1); presence of coronary thrombus from atrial fibrillation (n=3); thrombolysis before angiography (n=4); and missing data (n=52). The left 426 patients (aged 64.7±11.7 years; 81% men) were included in our analysis and categorized according to tertiles of EMR on admission into: T1 group (EMR <0.06, n=141), T2 group (0.06≤ EMR ≤0.18, n=140), and T3 group (EMR >0.18, n=145).
The study protocol was approved by our institutional ethics committee (Ethic Approval: Bro12-030). All patients provided informed consent.
Study definitions
STEMI was defined as typical chest pain lasting >30 minutes but <12 h with documented ST-segment elevation ≥1 mm in ≥2 contiguous leads or new left bundle branch block and elevated myocardial infarction markers (15). One-month and long-term all-cause mortality, defined as death from any reason during the observational period (29.5±3.5 days and 14.1±7.8 months, respectively) post P-PCI, were primary study outcomes. Major adverse cardiac event (MACE) included all-cause mortality, target vessel revascularization (TVR), and myocardial reinfarction. TVR was defined as any kind of revascularization of the initial target vessel, including coronary artery bypass graft (CABG) surgery and PCI. Myocardial reinfarction was recorded according to the third universal definition (16).
Data collection
The following demographic and clinical data were recorded: age, sex, blood pressure, medical history, chest pain duration and Killip class. The follow-up data were achieved by telephone. Blood samples were drawn on admission for the measurement of leucocytes, cardiac troponin T (cTNT), serum creatinine (Scr) and C-reactive protein (CRP) using a Sysmex XS 500i auto analyzer (Sysmex, Kobe, Japan). The blood samples were drawn again on the next morning for reviewing latter indexes and measuring serum lipid levels. All patients finished ultrasonic cardiogram (UCG) testing and the first result was recorded. The value of EMR was calculated by dividing eosinophil count by monocyte count.
Coronary angiography
All patients received dual antiplatelet therapy with aspirin (300 mg) and clopidogrel (300 mg) or ticagrelor (180 mg) before PCI. The statin and beta-blocker agents were prescribed to all the individuals if without contraindication. A team of at least two experienced cardiologists performed PCIs. The choices of approach, technique, and equipment were left to the operator. The interventional success was defined as the achievement of <20% diameter stenosis with Thrombolysis in Myocardial Infarction (TIMI) 3 flow in the target vessel. All angiographic and procedural details were judged and recorded by the operators and assistants who were blind to the study design and subsequent data analysis. All cases received standard perioperative hospital care.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) when normally distributed as per the Kolmogorov-Smirnov test or otherwise as median (interquartile range). Categorical variables were expressed as number (%). Continuous variables were compared by the Mann-Whitney U test and categorical variables were compared by Mantel-Haenszel test. The relationship between EMR and other variables was assessed using Spearman bivariate correlation analysis. Univariate analysis was applied to evaluate the effect of different variables on the all-cause mortality. Suspected variables or variables with P<0.1 in univariate analysis were included into multivariate Cox proportional hazards model. Collinearity diagnostics was finished before the multivariable analysis, whose results were presented as hazard ratios (HR) and 95% confidence intervals (95% CI). Survival curves were analyzed using Kaplan-Meier estimation and they were compared using the log-rank test. Receiver operating characteristic curve analysis was performed to determine the best cut-off value for predicting all-cause mortality and the sensitivity as well as the specificity of EMR. P value <0.05 was considered statistically significant. All data analyses were conducted by SPSS 22.0 software (IBM SPSS Statistics, USA).
Results
Patient baseline and clinical characteristics
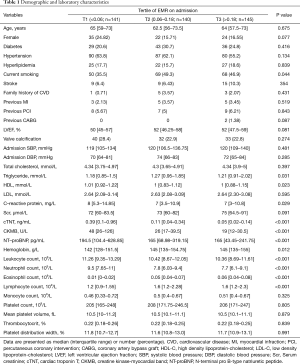
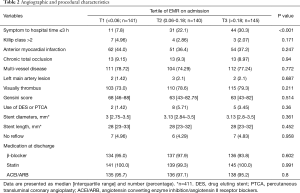
The baseline characteristics of the study population were provided in Tables 1,2. Compared with the T2 and T3 group, the T1 group had a higher level of high density lipoprotein-cholesterol (HDL-C), creatine kinase-myocardial band (CK-MB), cTNT, and neutrophil count, whereas a lower level of N-terminal pro b-type natriuretic peptide (NT-proBNP), hemoglobin, eosinophil count, and lymphocyte count. Besides, there was a higher proportion of patients with symptom-to-hospital time ≤3 h in the T1 group. There was no statistically significant difference in terms of other analyzed demographic, laboratory, and angiographic features.
Full table
Full table
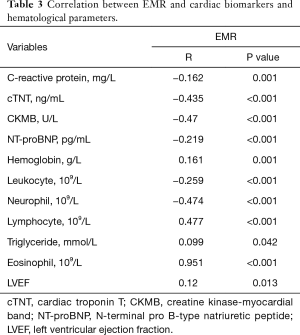
EMR was negatively correlated with cTNT, CK-MB, CRP, and NT-proBNP levels, and neutrophil, leukocyte counts. However, EMR had a positive and strong correlation with lymphocyte and eosinophil count, a positive and weak correlation with left ventricular ejection fraction (LVEF), and hemoglobin and triglyceride levels (Table 3).
Full table
Clinical outcomes and EMR
One-month and long-time all-cause mortality rate was 4.25% and 7.09% in the T1 group, 2.14% and 2.9% in the T2 group, and 0% and 0.7% in the T3 group [P (1-month) =0.012; P (long-term) =0.003, Table 4]. Other outcomes like TVR and myocardial reinfarction were comparable among groups, as were in-hospital cost and duration.

Full table
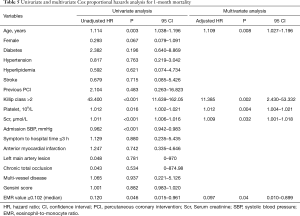
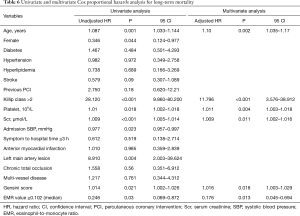
In multivariate Cox proportional hazards analysis to determine the correlation factors of all-cause mortality, EMR (hazard ratio =0.097; 95% CI, 0.010–0.899; P=0.04), age, Scr, platelet count, and Killip class were independently associated with one-month mortality (Table 5). In addition, EMR (hazard ratio =0.176; 95% CI, 0.045–0.694; P=0.013), age, Scr, platelet count, Killip class, and Gensini score were independently correlated with long-term mortality (Table 6). As several laboratory parameters such as CRP (r=−0.162; P=0.001), cTNT (r=−0.435; P<0.001), CK-MB (r=−0.47; P<0.001), eosinophil count (r=0.951; P<0.001), NT-pro BNP (r=−0.219; P<0.001), leukocyte (r=0.259; P<0.001), hemoglobin level (r=0.161; P=0.001) correlated with EMR, they were not included into multivariate analysis.
Full table
Full table
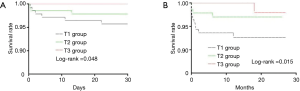
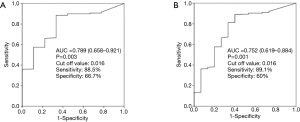
Both Kaplan-Meier survival curves for 1-month and long-term all-cause mortality (Figure 2) showed significant difference among groups. Apparently, the T3 group had a higher 1-month and long-term survival rate. In receiver operating characteristic curve analysis, the area under the curve of EMR for the prediction of one-month and long-term mortality was 0.789 (95% CI, 0.658–0.921) and 0.752 (95% CI, 0.619–0.884) (Figure 3).
Discussion
Our study demonstrated the association of EMR on admission and the 1-month and long-term total mortality in patients with STEMI who were treated with P-PCI. To the best of our knowledge, this is the first study to introduce EMR and prove the lower EMR was associated with higher one-month and long-term mortality in STEMI patients who underwent P-PCI.
In the previous studies, researchers have accumulated lots of evidence to illustrate the relationship between monocytes and pathological process of STEMI. First, monocytes are key cells in the process of atherosclerosis. The dysfunction of endothelial cells will lead to the recruitment of inflammatory cells in the arterial wall (17). After the recruitment, some monocytes can be activated into macrophages. The monocytes and macrophages subsequently release more inflammatory factors such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) attracting more inflammatory cells (18). Several clinical studies have showed that elevated monocytes were associated with left ventricular dysfunction in STEMI patients, which suggests that monocytes might affect the process of left ventricular remodeling (19). Erkol et al. also found that inhibiting monocytes gathering to infarcted myocardium might improve ventricular function (20).
An emerging finding of eosinophils is its effect on regulating the inflammation progress. Eosinophils act an important role in the initiation, progression, and rupture of thrombus. Eosinophils help platelets adhering to the injured vessel wall (21), and they can release immunosuppressive cytokines like IL-10, IL-4, and IL-13 which are suggested to modulate the inflammatory response (22). Atherosclerotic plaques rupture, a basic inflammatory pathogenesis of STEMI, involves the infiltration of eosinophils into the infarcted myocardium (14). In contrast to other inflammatory parameters, eosinophil was seldom discussed. Jiang found that circulatory eosinophils reflected the extent of myocardial infarction (23). Besides, eosinophils were found to be a useful biomarker for risk stratification of CAD patients and predicting 6-month mortality (24).
As the single inflammatory cell is unable to summarize the overall systematic inflammation, new indexes were proposed by combining different subtypes of the leukocyte. NLR (25), LMR (9,26) and eosinophil to leukocyte ratio (27) were reported to be independently associated with MACEs in STEMI patients with P-PCI. EMR is a totally new parameter. We found that a lower EMR was associated with a higher risk after STEMI, which may result from an increased monocyte count or a decreased eosinophil count. However, in our study, the monocyte count was comparable among the three groups and EMR values positively and strongly correlated with eosinophil counts. In this case, we speculated that a lower EMR may represent a reduction in eosinophil count. The low eosinophil count may due to the infiltration of eosinophils into the infarcted myocardium and coronary thrombi, which resulted in the decrease in peripheral circulating eosinophils (14). Besides, an increase in the cortisol concentration caused by an acute stress response to STEMI may also lower the peripheral eosinophil count (27). Both eosinophils and monocytes are inflammation and immune cells and related with immunosuppressive cytokines. Eosinophils were found to be able to lower the viability or activation of T cells (28).
In the present study, we demonstrated that a lower EMR on admission was associated with higher one-month and long-term mortality in patients with STEMI who underwent primary PCI. Besides, EMR was negatively related with cTNT and CK-MB. Thus, to some extent, EMR might have the potentiality to indicate the infarct size of the myocardium. Moreover, the Scr level on admission was independent risk factor of long-term total mortality which was in accord with the previous studies (29). Similarly, we also notice the relationship between the platelet count and 1-month as well as long-term mortality. Age, another parameter which was suggested to be associated with both 1-month and long-term mortality, was already widely used as a predictive factor in various prognosis analysis models (30).
Conclusions
We demonstrated that a lower EMR on admission was associated with higher 1-month and long-term mortality in patients with STEMI who underwent primary PCI. EMR could be a simple, useful, and inexpensive marker for risk stratification of STEMI patients.
Limitations
First, it was a single-center observational with inherent bias. Second, patients with very severe disease who died before undergoing coronary artery angiography were missed. Third, some patients were poorly compliant with use of prescribed medications. Larger-scale, prospective, and randomized clinical trials are required to confirm our findings.
Acknowledgements
We appreciate the collaboration of the co-investigators and the guidance of the correspondence author.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by our institutional ethics committee (Ethic Approval: Bro12-030). All patients provided informed consent.
References
- Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet 2017;389:197-210. [Crossref] [PubMed]
- Liu HL, Yang Y, Yang SL, et al. Administration of a loading dose of atorvastatin before percutaneous coronary intervention prevents inflammation and reduces myocardial injury in STEMI patients: a randomized clinical study. Clin Ther 2013;35:261-72. [Crossref] [PubMed]
- Carrick D, Haig C, Rauhalammi S, et al. Pathophysiology of LV Remodeling in Survivors of STEMI: Inflammation, Remote Myocardium, and Prognosis. JACC Cardiovasc Imaging 2015;8:779-89. [Crossref] [PubMed]
- Ghaffari S, Nadiri M, Pourafkari L, et al. The predictive Value of Total Neutrophil Count and Neutrophil/Lymphocyte Ratio in Predicting In-hospital Mortality and Complications after STEMI. J Cardiovasc Thorac Res 2014;6:35-41. [PubMed]
- Chiva-Blanch G, Bratseth V, Ritschel V, et al. Monocyte-derived circulating microparticles (CD14(+), CD14(+)/CD11b(+) and CD14(+)/CD142(+)) are related to long-term prognosis for cardiovascular mortality in STEMI patients. Int J Cardiol 2017;227:876-81. [Crossref] [PubMed]
- Gijsberts CM, Ellenbroek G, Ten BM, et al. Effect of Monocyte-to-Lymphocyte Ratio on Heart Failure Characteristics and Hospitalizations in a Coronary Angiography Cohort. Am J Cardiol 2017;120:911-6. [Crossref] [PubMed]
- Sosnovik DE, Nahrendorf M. Cells and iron oxide nanoparticles on the move: magnetic resonance imaging of monocyte homing and myocardial inflammation in patients with ST-elevation myocardial infarction. Circ Cardiovasc Imaging 2012;5:551-4. [Crossref] [PubMed]
- Clemente C, Rius C, Alonso-Herranz L, et al. MT4-MMP deficiency increases patrolling monocyte recruitment to early lesions and accelerates atherosclerosis. Nat Commun 2018;9:910. [Crossref] [PubMed]
- Wang Q, Ma J, Jiang Z, et al. Association of lymphocyte-to-monocyte ratio with in-hospital and long-term major adverse cardiac and cerebrovascular events in patients with ST-elevated myocardial infarction. Medicine (Baltimore) 2017;96. [Crossref] [PubMed]
- Murat SN, Yarlioglues M, Celik IE, et al. The Relationship Between Lymphocyte-to-Monocyte Ratio and Bare-Metal Stent In-Stent Restenosis in Patients With Stable Coronary Artery Disease. Clin Appl Thromb Hemost 2017;23:235-40. [Crossref] [PubMed]
- Ayça B, Akin F, Celik O, et al. Neutrophil to Lymphocyte Ratio is Related to Stent Thrombosis and High Mortality in Patients With Acute Myocardial Infarction. Angiology 2015;66:545-52. [Crossref] [PubMed]
- Kurtul S, Sarli B, Baktir AO, et al. Neutrophil to lymphocyte ratio predicts SYNTAX score in patients with non-ST segment elevation myocardial infarction. Int Heart J 2015;56:18-21. [Crossref] [PubMed]
- Núñez J, Nunez E, Bodi V, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron Artery Dis 2010;21:1-7. [Crossref] [PubMed]
- Cowan MJ, Reichenbach D, Turner P, et al. Cellular response of the evolving myocardial infarction after therapeutic coronary artery reperfusion. Hum Pathol 1991;22:154-63. [Crossref] [PubMed]
- Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619. [Crossref] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. [Crossref] [PubMed]
- Gerszten RE, Friedrich EB, Matsui T, et al. Role of phosphoinositide 3-kinase in monocyte recruitment under flow conditions. J Biol Chem 2001;276:26846-51. [Crossref] [PubMed]
- Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr 2005;25:151-74. [Crossref] [PubMed]
- Jugdutt BI. Monocytosis and adverse left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol 2002;39:247-50. [Crossref] [PubMed]
- Erkol A, Oduncu V, Turan B, et al. Neutrophil to lymphocyte ratio in acute ST-segment elevation myocardial infarction. Am J Med Sci 2014;348:37-42. [Crossref] [PubMed]
- Avramakis G, Papadimitraki E, Papakonstandinou D, et al. Platelets and white blood cell subpopulations among patients with myocardial infarction and unstable angina. Platelets 2007;18:16-23. [Crossref] [PubMed]
- Jacobsen EA, Taranova AG, Lee NA, et al. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol 2007;119:1313-20. [Crossref] [PubMed]
- Jiang P, Wang DZ, Ren YL, et al. Significance of eosinophil accumulation in the thrombus and decrease in peripheral blood in patients with acute coronary syndrome. Coron Artery Dis 2015;26:101-6. [Crossref] [PubMed]
- Toor IS, Jaumdally R, Lip GY, et al. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb Res 2012;130:607-11. [Crossref] [PubMed]
- Balta S, Demirkol S, Unlu M, et al. Neutrophil to lymphocyte ratio may be predict of mortality in all conditions. Br J Cancer 2013;109:3125-6. [Crossref] [PubMed]
- Kiris T, Celik A, Varis E, et al. Association of Lymphocyte-to-Monocyte Ratio With the Mortality in Patients With ST-Elevation Myocardial Infarction Who Underwent Primary Percutaneous Coronary Intervention. Angiology 2017;68:707-15. [Crossref] [PubMed]
- Konishi T, Funayama N, Yamamoto T, et al. Prognostic Value of Eosinophil to Leukocyte Ratio in Patients with ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J Atheroscler Thromb 2017;24:827-40. [Crossref] [PubMed]
- Odemuyiwa SO, Ghahary A, Li Y, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol 2004;173:5909-13. [Crossref] [PubMed]
- Wang CH, Zhang SY, Fang Q, et al. Renal Dysfunction and hsCRP Predict Long-term Outcomes of Percutaneous Coronary Intervention in Acute Myocardial Infarction. Am J Med Sci 2015;349:413-20. [Crossref] [PubMed]
- De Maria GL, Fahrni G, Alkhalil M, et al. A tool for predicting the outcome of reperfusion in ST-elevation myocardial infarction using age, thrombotic burden and index of microcirculatory resistance (ATI score). Eurointervention 2016;12:1223-30. [Crossref] [PubMed]