Patient screening for early detection of aortic stenosis (AS)—review of current practice and future perspectives
Introduction
The burden of heart valve disease (VHD) is rising due to an increased life expectancy in the elderly population (1-4). Degenerative aortic stenosis (AS) is associated with a high mortality if diagnosed late and if no valve replacement therapy is performed (5). In patients with an established diagnosis of AS however, only two-thirds of those meeting guideline recommendations for valve replacement therapy actually receive treatment, with failure to intervene due mostly to an overestimation of the risks involved, underestimation of symptoms or misclassification of the severity of stenosis (6,7). Recent data shows that most patients are only diagnosed when they develop symptoms, as this precipitates referral of the patient for echocardiography (8). Valve replacement therapy may have been indicated in a subset of these patients even before the onset of symptoms, not only in those with impaired left ventricular ejection fraction (LVEF <50%) or in those whom exercise testing unmasks symptoms but also in those with very severe AS (Vmax >5.5 m/s), severe valve calcification and evidence of fast progression, pulmonary hypertension or markedly elevated brain natriuretic peptide (BNP) levels (9). Furthermore, there is accumulating evidence for the clinical benefit of early intervention in all patients with asymptomatic severe AS (10,11) as symptom-onset may well represent an arbitrary timepoint in the course of the disease (12). Certainly, outcomes deteriorate in proportion to myocardial response, particularly where there is replacement fibrosis, and recovery is sub-optimal in these patients (13). As AS follows a long indolent course over years during which patients are unaware of their condition and mortality rates increase dramatically soon after onset of symptoms, both patient and physician awareness need to be increased and methods of early diagnosis rates and referral need to be improved (14). The first presentation of AS is frequently in the primary care setting and, as a result, family physicians/general practitioners (GPs) play a key role in timely diagnosis and referral of patients with suspected VHD (15). The following article tries to evaluate the status and future perspectives of patient screening for AS.
Clinical aspects and burden of AS
Clinical presentation and epidemiology
Moderate or severe VHD is present in approximately 13% of patients aged ≥75 years, with the most common conditions being AS and mitral regurgitation (1). According to a recently published meta-analysis, 12.4% of subjects aged >75 years suffer from AS and 3.4% from severe AS (4). AS develops over years and shares commonalities with the pathophysiology of atherosclerosis, where an inflammatory process leads to progressive leaflet-thickening and calcification (sclerosis) with an associated narrowing of the aortic valve orifice and increase in afterload (16). Patients with bicuspid aortic valves, the most common congenital cardiac anatomy (with a prevalence of approximately 1%), are prone to accelerated valve calcification and make up the majority of the population undergoing valve replacement therapy below the age of 60 years (17). Based on echocardiographic findings (transvalvular gradient, flow velocity and effective orifice area), the degree of AS usually progresses as an asymptomatic condition from mild or moderate to severe. The occurrence of typical symptoms in patients with severe AS (shortness of breath, angina and dizziness/syncope) is usually considered the appropriate timepoint for intervention as the maximum life expectancy in these patients is about 5 years and disease mortality exceeds the peri-operative risk of valve replacement intervention (5,9). Although most patients are symptomatic by the time of diagnosis, a major problem is that the symptoms patients’ typically exhibit are not specific for AS, and are frequently attributed to other common conditions of the elderly, especially chronic obstructive pulmonary disease (COPD), ischaemic heart disease or neurocardiogenic syncope (4,18). The threshold for screening and referral, therefore, must be low.
Burden of AS to the healthcare system
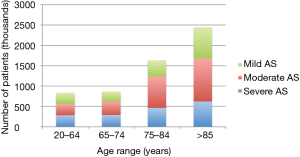
AS is associated with a significant financial burden to healthcare systems. Published evidence on AS diagnosis rates are sparse, but assumptions can be made based on AS prevalence data from population-based studies. The estimated number of cases of severe, moderate and mild AS in Europe is shown in Figure 1. In the US, AS accounts for an estimated 1.5 million patients—about 500,000 patients with severe stage disease and 250,000 patients with symptomatic disease (19). This burden of disease will increase further with ageing of the population. According to a recent analysis by Moore et al., aortic valve disease is associated with an estimated 10.2 billion USD incremental annual healthcare spending, including 10,816 USD/year per asymptomatic patient and 12,789 USD/year per symptomatic patient in the US (20). A European perspective on direct costs of AS is provided by Veronesi et al., who compared the cost of illness for a period of 2 years before an index hospitalisation for AS with costs of the two subsequent years in a cohort of 919 patients hospitalised between 2007–2011 in Italy. In patients who received surgical valve replacement, direct costs decreased from 28,365 EUR to 8,002 EUR after the intervention, indicating a significant cost burden of untreated severe AS patients (21). Patients with severe AS are costly to care for due to repeated hospitalisation and the need for heart failure therapies (cheap generics) (22).
AS: awareness and diagnosis rates
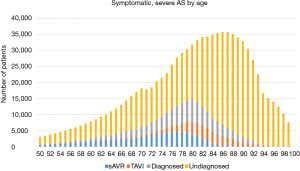
The biggest catalyst for the implementation of successful disease screening measures is to increase public, patient and physician awareness of AS. According to a recently published survey in almost 9,000 subjects aged ≥60 years across nine European countries, only 2% of the respondents expressed concerns about VHD. In contrast, 28% of study patients were concerned about cancer and 25% about Alzheimer’s disease. When patients were asked about their knowledge of AS, 92% of respondents had no knowledge of the condition or provided an incorrect definition. Interestingly, when provided with information about AS, about 5% of patients reported greater level of concern and had recognised symptoms in themselves (23). The low awareness of AS symptoms and their importance was confirmed in another study of patients after diagnosis with severe AS, in which only 27–56% reported awareness of 1 of 3 main AS symptoms (shortness of breath, angina and dizziness/syncope), 69% reported awareness of two symptoms and only 16% reported awareness of all three symptoms (24). As the occurrence of symptoms usually defines the timing of valve replacement therapy in patients with severe AS, a low level of symptom awareness in previously diagnosed and undiagnosed patients could contribute to undertreatment or a significant delay in treatment and premature death (Figure 2). With this in mind, a variety of patient awareness campaigns are underway to help patients with the early identification of typical AS symptoms (25).
Diagnostic evaluation
Patients presenting with symptoms of advanced AS, including angina, dyspnoea and syncope, have a much higher mortality rate than asymptomatic patients. When patients present as a medical emergency, an initial diagnostic work-up should include an electrocardiogram, complete blood count, basic metabolic profile, coagulation studies, troponin, BNP and a chest radiograph (26). Essential diagnostic tests for AS, which can be performed in any medical environment, are auscultation and transthoracic echocardiography (TTE), which are discussed in detail below.
Auscultation
Chest auscultation represents an important tool to support the diagnosis of suspected heart valve disease. However, the widespread use of echocardiography over the past decades has led to a decline of the utility of auscultation in primary care institutions. AS characteristics, as detected by auscultation, are mid-systolic, diamond-shaped murmur, which radiates along the aortic outflow tract (right-sided subclavian region) with a maximum intensity in the area of the right upper sternal border. In some patients, a characteristic systolic murmur can also be detected at the level of the apex (Gallavardin phenomenon), which could erroneously raise suspicion of mitral valve disease. Progressive AS is associated with changes in the characteristics of the S2 (paradoxical split or softening of murmur). There appears to be a suboptimal correlation between the intensity of the systolic murmur and the severity of AS, as systolic or diastolic function might deteriorate over time, resulting in a decrease of the ejection fraction (27). A high-pitched systolic ejection click can be detected in some patients with bicuspid aortic valves. Unfortunately, there are very few published data on the relationship between findings on heart murmurs and their echocardiographic correlation with AS. In a subcohort of the OxValve population cohort study, 251 patients without previous diagnosis of VHD underwent cardiac auscultation by two GPs and findings were compared to TTE in an investigator-blinded evaluation. Auscultation was shown to have a sensitivity of only 32% and specificity of 67% for diagnosing mild VHD, which increased to 43% and 69% for significant VHD, respectively (28). Interestingly, clinical examination—despite its limitations—may have a predictive value in terms of outcome in AS. Bodegard et al. studied a cohort of apparently healthy middle-aged men (aged 40–59 years), including 23.4% subjects with systolic murmurs, followed for up to 35 years. In those subjects with a low-grade murmur, there was a 4.7-fold, age-adjusted increase in risk of aortic valve replacement and an 89.3-fold increase in risk for those with moderate-grade murmur (29). When asked about cardiac auscultation performed by their GP, approximately half of the male patients and one-third of the female patients (<60 years of age) reported auscultation at every, every second or every third visit (23). These data were confirmed by data from a recent survey in 153 GPs from France, Germany and the UK, where only 62% performed auscultation routinely in elderly patients. In the UK and Germany, cardiac auscultation in symptomatic patients was only performed in 38% and 40% of elderly patients, respectively (30). These findings suggest that there may be a significant proportion of patients in any practice who have valve disease that is undetected and they are not, therefore, referred for echocardiography (31).
Echocardiography
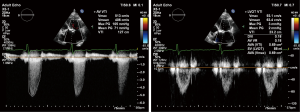
Echocardiography is the primary tool for diagnosis, evaluation and risk stratification of valve disease. As most valve pathology develops gradually over time, echocardiographic screening can deliver accurate detection and risk stratification of patients at all stages of disease. Even when aortic sclerosis rather than stenosis is found (see Figure 3), which is present in one out of four persons aged ≥65 years and nearly half of patients aged >80 years (32,33), this may lead to improved patient care. Although previously thought to be benign, the degree of calcification in aortic sclerosis both predicts risk of progression to AS and also reflects increased cardiovascular risk due to atherosclerosis (34-37). Furthermore, systematic screening with echocardiography in the community not only identifies valve disease but also cardiomyopathy and heart failure. Based on echocardiography performed in >6,000 randomly selected patients aged >45 years from 16 GP-practices in the UK, 26% of patients were diagnosed with VHD and ~3% of patients with unheralded heart failure (38).
Patient referral
Once a patient has been diagnosed with severe AS, the treating clinician should adhere to treatment guidelines and refer the patient for intervention. However, several studies have reported non-adherence to clinical guidelines and have cited discrepancies between the decision to treat patients and current scientific recommendations occurring in up to 42% of patient cases (7,39-42). Reasons cited for non-adherence for to treatment guidelines included over and under use of AS interventions and lack of diagnostic data to make an informed treatment decision (40). Results of the IMPULSE Study, which is gathering data over a 12-month period on physician decisions to treat patients with newly diagnosed severe AS, is eagerly awaited and could help to serve the creation of a clinical care pathway to improve the timely management of these patients (8).
Feasibility and effectiveness of as screening in general practice
General considerations
Systematic disease screening in the primary care setting appears to be a valuable opportunity to identify patients with VHD and, in particular, AS (31). In Germany, 43% of 420,000 practicing physicians work in ambulatory care and each of the about 60,000 GPs is visited by an average of 243 patients/week (43). In the US, 52% of all patient visits are recorded in the primary care setting. In the age-group of patients aged ≥75, 89% of patients had visited their physician at least once within the previous 6 months (86% of patients in the age group of 65–74 years) (44). These numbers indicate a high intensity of patient-GP interaction in the elderly population and these interactions are, therefore, a significant opportunity for the detection of undiagnosed VHD. However, there appears to be an impact of patient age on the patient-physician interaction in primary care, as older patients receive less counselling, are asked fewer questions, are less often provided with health education and are more often monitored for treatment compliance only (45). Furthermore, data from the US National Ambulatory Care Survey (1997–2010) reveals, that medical specialist primary care physicians take care of older patients (mean age 61 years), managing mostly chronic disease (51%), whereas generalist primary care physicians treat younger patients (mean age 55.4 years) dedicating their time to the care of new health problems (40.5%). Generalist primary physicians might not, therefore, encounter in daily practice a high number of patients where VHD is likely to be present (46). Still, disease screening measures for cardiovascular and non-cardiovascular conditions, such as arterial hypertension, diabetes or prostate- and colorectal cancer are well-established in the primary care setting as non-invasive diagnostic tools are readily available and screening measures are not considered as time-consuming or costly (47-50). However, the usefulness of any disease screening measure in an elderly population needs to be thoroughly assessed based on the expected disease prevalence in a particular age group, costs and feasibility of screening (for example, level of invasiveness and time-consumption) as well as an evaluation of potential patient benefits from existing treatment options for a particular condition, but also in light of patient comorbidities, expected effects of treatment on life-expectancy and quality of life. Generally, a high level of sensitivity and specificity is required to ensure that a condition is not missed or erroneously suspected in a considerable number of patients—with an associated impact on patient health and costs (51). Data from Germany suggests, that patients aged ≥65 years of age make up 32% of all visits to primary care physicians (19% for patients aged 65–74 years and 13% for patients aged ≥75 years), indicating that a GP in Germany has about 320 patient encounters per month with a patient aged >65 years and about 120 encounters with a patient aged ≥75 years of age (43,52). Based on an AS-prevalence rate of 12.4% in subjects >75 years of age, an estimated 15 patients with any grade of AS and four patients with severe AS could be potentially identified with dedicated measures on a monthly basis in general practice (4).
Screening for AS symptoms and cardiac auscultation
Systematic screening for symptoms in elderly patients represents the most simplistic approach to the diagnosis of advanced stages of AS. Even though, typical AS symptoms can be of multiple origin, it has been shown, that dizziness/pre-syncope results in the majority of cases from cardiovascular conditions (57%) and less frequently from other reasons, such as adverse drug effects (23%), peripheral vestibular disease (14%) and psychiatric illness (14%) (53). Dyspnoea represents the reason for ~1% of all non-procedure-related visits of patients >65 years of age to GPs in Germany (52). Dyspnoea in general practice can be attributed in about 50% of cases to acute respiratory infections, bronchial asthma or COPD, but only in about 4–6% of cases to heart failure and up to 2% of cases to cardiac arrhythmias, conditions which do potentially coincide with AS (54). Patient visits in general practice are due to chest pain/angina in up to 3% of cases, the majority of which occur in the patient group of 45–64 years of age (39.3%), followed by patients aged 65–74 years (18.5%) and patients aged >75 years (12.2%). With up to almost half of all visits caused by musculoskeletal problems, cardiovascular conditions represent the second most frequent reason for chest pain/angina (55). As chest pain in patients with AS is usually the result of reversible myocardial ischemia due to macro-/microvascular disease, the diagnostic pathway needs to rule out other pathological conditions first, in order to establish the diagnosis of AS as the underlying cause (56).
For decades, stethoscopes have been essential tools in clinical patient evaluation. However, data from the US, Canada and UK indicate, that a correct assessment of a heart murmurs was only made in about 25% of patients by internal medicine residents, indicating a lack of emphasis for auscultation in teaching and practice (57,58). On the other hand, accurately diagnosed abnormal auscultatory findings are not necessarily confirmed by echocardiography as shown by McBrien et al. In almost 4,000 patients undergoing hip surgery, a systolic murmur was detected in 22.7% of cases, but a diagnosis of AS could be only confirmed in 30% of patients by echocardiography (65.4% mild, 23.5% moderate and 11% severe) (28,59). Even though the overall sensitivity of cardiac auscultation appears low for diastolic murmurs (0.21–1.00), sensitivity for systolic murmurs is higher (0.67–1.00), supporting the usefulness of auscultation as a relevant diagnostic tool particularly for the detection of patients with AS (58). Another relevant limitation of the utility of cardiac auscultation in general practice is the timing of a patient-physician interaction in daily clinical routine, which appears to be in the range of 10–20 minutes in 75% of visits (60). As auscultation mandates an appropriate preparation—such as a warm/quiet room, an examination table/bed and a sufficiently exposed patient chest—and the short patient-physician interaction does not necessarily support a skillful auscultation with the objective to identify VHD (61).
Echocardiographic AS-screening—innovative concepts
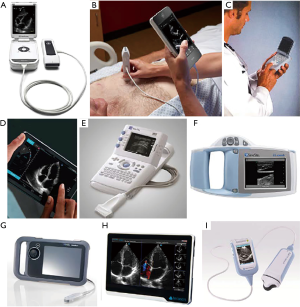
As growth of healthcare expenditure is on the agenda of healthcare providers and payors, the overuse of costly diagnostic measures represents one of the areas of concern. On the other hand, the routine use of handy echo devices like the VScan should be on the agenda of medical education early on just like using the stethoscope. But, in most countries, performance of imaging is increasing at a rate of >10%/year (62) and cost of a TTE varies significantly between £66–425 in the UK and >2,000 USD in the US (63,64). Furthermore, there is often limited capacity for performing an echocardiogram in a timely manner. Even though the majority of echocardiographic studies ordered in the primary care setting appear to be appropriate, only about 20% of patient abnormalities are detected and only 2.5% findings were consistent with the suspected diagnosis and led to a change in patient management (65). Generally, echocardiographic screening should be restricted to symptomatic patients in order to increase the likelihood of detecting relevant cardiac pathologies (66). As degenerative AS is a disease of the elderly, systematic echocardiographic evaluations should mainly focus on subjects aged 65 or older, when the prevalence of AS starts to rise significantly (1). Routine-screening, therefore, could be targeting facilities such as nursery homes or institutions of assisted living, where mobile heart scanning clinics could be run by certified nurse practitioners or non-medical health professionals who are educated in echocardiography (67-69). Another promising approach represents patient screening during dedicated flu vaccinations in primary care, where the value of echocardiography screening is being tested in ongoing studies. For these settings, various mobile (miniaturised) echocardiogram devices are commercially available, allowing for earlier disease detection, triage improvement and facilitation of patient referral (Figure 4). However, even though these devices allow for a limited, but quite reliable echocardiography assessment of some conditions, the evaluation of VHD still represents one of the most limited functions in terms of correlation with TTE, mainly due to a lack of spectral Doppler and a lower image resolution (71). On the other hand, studies suggest the highest level of sensitivity for the detection of valvular stenosis with these hand-held devices (72).
As limitations in the accessibility to an echocardiogram performed by an experienced echocardiographer do exist, novel concepts of machine learning have shown promising results. Learning algorithms introduced into medical image analysis software could help not only to locate standard heart views but discriminate physiological from pathological conditions, thereby facilitating accurate image acquisition and interpretation in examinations performed by less-experienced personnel (73). While currently existing applications mainly enable an assessment of ejection fraction, myocardial wall motion and myocardial strain, their usefulness in the assessment of VHD has been only described for mitral valve disease so far (74,75).
Conclusions
Severe AS is a common, but serious, complication that affects more than one million people aged ≥75 years in Europe and with an aging population the number of cases of severe AS will keep increasing (4). Without valve replacement therapy, patients with severe, symptomatic AS face an average life expectancy of 2–3 years and an increased risk of sudden death (76-78). More can, and needs, to be done to mitigate the impact of AS on society and the associated financial burden on healthcare resources. The first challenge is to increase the timely diagnoses of AS. Possible avenues to achieve this include the implementation of a screening programme in the elderly during visits to GPs or in residential care and/or raising awareness of disease symptoms in the patients themselves to open dialogues with their GPs/clinicians. Once diagnosed, physicians should follow treatment guidelines to ensure approach patient management, although currently only a proportion of patients are managed in this manner (40,79), but the creation of clear care pathways might improve adherence to treatment regimens.
Acknowledgements
None.
Footnote
Conflicts of Interest: M Thoenes and J Kurucova are employees of Edwards Lifesciences. The other authors have no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. [Crossref] [PubMed]
- Lindroos M, Kupari M, Heikkila J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21:1220-5. [Crossref] [PubMed]
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002-12. [Crossref] [PubMed]
- Kapadia SR, Leon MB, Makkar RR, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2485-91. [Crossref] [PubMed]
- van Geldorp MW, van Gameren M, Kappetein AP, et al. Therapeutic decisions for patients with symptomatic severe aortic stenosis: room for improvement? Eur J Cardiothorac Surg 2009;35:953-7; discussion 957. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Frey N, Steeds RP, Serra A, et al. Quality of care assessment and improvement in aortic stenosis - rationale and design of a multicentre registry (IMPULSE). BMC Cardiovasc Disord 2017;17:5. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- The PARTNER 3 Trial - The safety and effectiveness of the SAPIEN 3 transcatheter heart valve in low risk patients with aortic stenosis (P3). ClinicalTrials.gov. 2018. Accessed 29 April 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT02675114
- TAVR forges ahead in PARTNER III for low-risk patients. PM360. 2016. Accessed 29 April 2018. Available online: https://www.pm360online.com/tavr-forges-ahead-in-partner-iii-for-low-risk-patients/
- Braunwald E. On the natural history of severe aortic stenosis. J Am Coll Cardiol 1990;15:1018-20. [Crossref] [PubMed]
- Chin CWL, Everett RJ, Kwiecinski J, et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. JACC Cardiovasc Imaging 2017;10:1320-33. [Crossref] [PubMed]
- Iivanainen AM, Lindroos M, Tilvis R, et al. Natural history of aortic valve stenosis of varying severity in the elderly. Am J Cardiol 1996;78:97-101. [Crossref] [PubMed]
- Helms AS, Bach DS. Heart valve disease. Prim Care 2013;40:91-108. [Crossref] [PubMed]
- Rajamannan NM. Valvular Heart Disease: A Companion to Braunwald's Heart Disease. In: Otto CM, Bonow RO. editors. Valvular Heart Disease: A Companion to Braunwald's Heart Disease. 3rd edition. 1600 John F. Kennedy Blvd, Ste 1800, Philadelphia, PA 19103-2899: Saunders, Elsevier, 2009:39-54.
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920-5. [Crossref] [PubMed]
- Cary T, Pearce J. Aortic stenosis: pathophysiology, diagnosis, and medical management of nonsurgical patients. Crit Care Nurse 2013;33:58-72. [Crossref] [PubMed]
- John Muir Health. Transcatheter Aortic Valve Replacement (TAVR): Facts and Figures. John Muir Health. 2018. Accessed 24 April 2018. Available online: https://www.johnmuirhealth.com/services/cardiovascular-services/intervention/transcatheter-aortic-valve-replacement/facts-and-figures.html
- Moore M, Chen J, Mallow PJ, et al. The direct health-care burden of valvular heart disease: evidence from US national survey data. Clinicoecon Outcomes Res 2016;8:613-27. [Crossref] [PubMed]
- Veronesi C, Beccagutti G, Corbo M, et al. Cost Of Illness In Aortic Stenosis Patients. Value Health 2015;18:A386. [Crossref] [PubMed]
- Brecker S, Mealing S, Padhiar A, et al. Cost-utility of transcatheter aortic valve implantation for inoperable patients with severe aortic stenosis treated by medical management: a UK cost-utility analysis based on patient-level data from the ADVANCE study. Open Heart 2014;1. [Crossref] [PubMed]
- Gaede L, Di Bartolomeo R, van der Kley F, et al. Aortic valve stenosis: what do people know? A heart valve disease awareness survey of over 8,800 people aged 60 or over. EuroIntervention 2016;12:883-9. [Crossref] [PubMed]
- Guerbaii RA, Fustier G, Ennezat PV, et al. Asymptomatic aortic stenosis: An assessment of patients' and of their general practitioners' knowledge, after an indexed specialized assessment in community practice. PLoS One 2017;12. [Crossref] [PubMed]
- Alliance for Aging Research. Listen to Your Heart. 2018. Accessed 24 April 2018. Available online: http://www.valvediseaseday.org/listentoyourheart/
- Gottlieb M, Long B, Koyfman A. Evaluation and Management of Aortic Stenosis for the Emergency Clinician: An Evidence-Based Review of the Literature. J Emerg Med 2018;55:34-41. [Crossref] [PubMed]
- Das P, Pocock C, Chambers J. The patient with a systolic murmur: severe aortic stenosis may be missed during cardiovascular examination. QJM 2000;93:685-8. [Crossref] [PubMed]
- Myerson S, Prendergast B, Gardezi S, et al. 136 Gp auscultation for diagnosing valvular heart disease. Heart 2017;103:A101-2. [Crossref]
- Bodegard J, Skretteberg PT, Gjesdal K, et al. Low-grade systolic murmurs in healthy middle-aged individuals: innocent or clinically significant? A 35-year follow-up study of 2014 Norwegian men. J Intern Med 2012;271:581-8. [Crossref] [PubMed]
- Webb J, Thoenes M, Chambers JB. Identifying heart valve disease in primary care: Differences between practice in Germany, France and the United Kingdom. Eur J Cardiovasc Med 2014;3:388-92.
- Bouma BJ, van der Meulen JHP, van den Brink RBA, et al. Variability in treatment advice for elderly patients with aortic stenosis: a nationwide survey in the Netherlands. Heart 2001;85:196-201. [Crossref] [PubMed]
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005;111:3316-26. [Crossref] [PubMed]
- Olsen MH, Wachtell K, Bella JN, et al. Aortic valve sclerosis relates to cardiovascular events in patients with hypertension (a LIFE substudy). Am J Cardiol 2005;95:132-6. [Crossref] [PubMed]
- Rosenhek R, Klaar U, Schemper M, et al. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199-205. [Crossref] [PubMed]
- Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142-7. [Crossref] [PubMed]
- Aronow WS, Ahn C, Shirani J, et al. Comparison of frequency of new coronary events in older subjects with and without valvular aortic sclerosis. Am J Cardiol 1999;83:599-600, A8.
- Nightingale AK, Horowitz JD. Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart 2005;91:1389-93. [Crossref] [PubMed]
- Davies M, Hobbs F, Davis R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001;358:439-44. [Crossref] [PubMed]
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005;26:2714-20. [Crossref] [PubMed]
- Iung B, Messika-Zeitoun D, Cachier A, et al. Actual management of patients with asymptomatic aortic valve disease: how practice fits with guidelines. Am Heart J 2007;153:696-703. [Crossref] [PubMed]
- Freed BH, Sugeng L, Furlong K, et al. Reasons for nonadherence to guidelines for aortic valve replacement in patients with severe aortic stenosis and potential solutions. Am J Cardiol 2010;105:1339-42. [Crossref] [PubMed]
- Chan RH, Shaw JL, Hauser TH, et al. Guideline Adherence for Echocardiographic Follow-Up in Outpatients with at Least Moderate Valvular Disease. J Am Soc Echocardiogr 2015;28:795-801. [Crossref] [PubMed]
- Baum E. Primary Care in Germany. European Forum for Primary Care. 2018. Accessed 24 April 2018. Available online: http://www.euprimarycare.org/column/primary-care-germany
- National Center for Health Statistics. Centers for Disease Contrl and Prevention. 2018. Accessed 24 April 2018. Available online: https://www.cdc.gov/nchs/fastats/physician-visits.htm
- Callahan EJ, Bertakis KD, Azari R, et al. The influence of patient age on primary care resident physician-patient interaction. J Am Geriatr Soc 2000;48:30-5. [Crossref] [PubMed]
- Edwards ST, Mafi JN, Landon BE. Trends and quality of care in outpatient visits to generalist and specialist physicians delivering primary care in the United States, 1997-2010. J Gen Intern Med 2014;29:947-55. [Crossref] [PubMed]
- Bramlage P, Pittrow D, Lehnert H, et al. Frequency of albuminuria in primary care: a cross-sectional study. Eur J Cardiovasc Prev Rehabil 2007;14:107-13. [Crossref] [PubMed]
- Balijepalli C, Bramlage P, Losch C, et al. Prevalence and control of high blood pressure in primary care: results from the German Metabolic and Cardiovascular Risk Study (GEMCAS). Hypertens Res 2014;37:580-4. [Crossref] [PubMed]
- Moebus S, Balijepalli C, Losch C, et al. Age- and sex-specific prevalence and ten-year risk for cardiovascular disease of all 16 risk factor combinations of the metabolic syndrome - A cross-sectional study. Cardiovasc Diabetol 2010;9:34. [Crossref] [PubMed]
- Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA 2014;311:1143-9. [Crossref] [PubMed]
- Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. Inhal Toxicol 2014;26:811-28. [Crossref] [PubMed]
- Frese T, Mahlmeister J, Deutsch T, et al. Reasons for elderly patients GP visits: results of a cross-sectional study. Clin Interv Aging 2016;11:127-32. [Crossref] [PubMed]
- Maarsingh OR, Dros J, Schellevis FG, et al. Causes of persistent dizziness in elderly patients in primary care. Ann Fam Med 2010;8:196-205. [Crossref] [PubMed]
- Frese T, Sobeck C, Herrmann K, et al. Dyspnea as the reason for encounter in general practice. J Clin Med Res 2011;3:239-46. [PubMed]
- Frese T, Mahlmeister J, Heitzer M, et al. Chest pain in general practice: Frequency, management, and results of encounter. J Family Med Prim Care 2016;5:61-6. [Crossref] [PubMed]
- Lumley M, Williams R, Asrress KN, et al. Coronary Physiology During Exercise and Vasodilation in the Healthy Heart and in Severe Aortic Stenosis. J Am Coll Cardiol 2016;68:688-97. [Crossref] [PubMed]
- Mangione S. Cardiac auscultatory skills of physicians-in-training: a comparison of three English-speaking countries. Am J Med 2001;110:210-6. [Crossref] [PubMed]
- Alam U, Asghar O, Khan SQ, et al. Cardiac auscultation: An essential clinical skill in decline. . Br J Cardiol 2010;17:8-10.
- McBrien ME, Heyburn G, Stevenson M, et al. Previously undiagnosed aortic stenosis revealed by auscultation in the hip fracture population--echocardiographic findings, management and outcome. Anaesthesia 2009;64:863-70. [Crossref] [PubMed]
- Peckham C. Medscape Physician Compensation Report 2016. Medscape. 2016. Accessed 24 April 2018. Available online: https://www.medscape.com/features/slideshow/compensation/2016/public/overview#page=1
- Chizner MA. Cardiac auscultation: rediscovering the lost art. Curr Probl Cardiol 2008;33:326-408. [Crossref] [PubMed]
- Marwick TH. Can we justify the cost of echocardiography? Lessons from outcomes research. Eur J Echocardiogr 2005;6:155-63. [Crossref] [PubMed]
- Two Views. What are the costs or price of echocardiograms? Two Views. 2017. Accessed 24 April 2018. Available online: https://two-views.com/echocardiogram/cost-price.html
- Simpson E, Stevenson M, Scope A, et al. Echocardiography in newly diagnosed atrial fibrillation patients: a systematic review and economic evaluation. Health Technol Assess 2013;17:1-263. v-vi. [Crossref] [PubMed]
- Bethge A, Penciu O, Baksh S, et al. Appropriateness vs value: Echocardiography in primary care. Clin Cardiol 2017;40:1212-7. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Duvall WL, Croft LB, Goldman ME. Can hand-carried ultrasound devices be extended for use by the noncardiology medical community? Echocardiography 2003;20:471-6. [Crossref] [PubMed]
- Lowery J, Hopp F, Subramanian U, et al. Evaluation of a nurse practitioner disease management model for chronic heart failure: a multi-site implementation study. Congest Heart Fail 2012;18:64-71. [Crossref] [PubMed]
- Foundation. BH. Expanding the scope of nursing with echocardiography. British Heart Foundation. 2018. Accessed 26 April 2018. Available online: https://www.bhf.org.uk/healthcare-professionals/articles/profile/expanding-the-scope-of-nursing-with-echocardiography
- Chamsi-Pasha MA, Sengupta PP, Zoghbi WA. Handheld Echocardiography: Current State and Future Perspectives. Circulation 2017;136:2178-88. [Crossref] [PubMed]
- Seraphim A, Paschou SA, Grapsa J, et al. Pocket-Sized Echocardiography Devices: One Stop Shop Service? J Cardiovasc Ultrasound 2016;24:1-6. [Crossref] [PubMed]
- Kitada R, Fukuda S, Watanabe H, et al. Diagnostic accuracy and cost-effectiveness of a pocket-sized transthoracic echocardiographic imaging device. Clin Cardiol 2013;36:603-10. [PubMed]
- Narula S, Shameer K, Salem Omar AM, et al. Machine-Learning Algorithms to Automate Morphological and Functional Assessments in 2D Echocardiography. J Am Coll Cardiol 2016;68:2287-95. [Crossref] [PubMed]
- Slomka PJ, Dey D, Sitek A, et al. Cardiac imaging: working towards fully-automated machine analysis & interpretation. Expert Rev Med Devices 2017;14:197-212. [Crossref] [PubMed]
- Jeganathan J, Knio Z, Amador Y, et al. Artificial intelligence in mitral valve analysis. Ann Card Anaesth 2017;20:129-34. [Crossref] [PubMed]
- Bach DS, Siao D, Girard SE, et al. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2:533-9. [Crossref] [PubMed]
- Bouma BJ, van Den Brink RB, van Der Meulen JH, et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart 1999;82:143-8. [Crossref] [PubMed]
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. [Crossref] [PubMed]


