Treatment of persistent bronchopleural fistula with a manually modified endobronchial stent: a case-report and brief literature review
Introduction
Bronchopleural fistulas (BPFs) carry significant morbidity (1,2) and mortality. Although surgery remains the cornerstone of care, no standard therapeutic approach to the management of BPFs has been established. Different BPF sizes and anatomical locations, along with variability in patient comorbidities often mandate conservative therapeutic strategies (1,3). The use of endobronchial stents has been described as an alternative when surgical approaches are limited (4,5). Stents protect the contralateral lung from spillage while allowing time for the BPF to heal. Moreover, these are more easily placed and can be better tolerated compared to surgery when patients have significant comorbidities (6). Data published describing the extent to which airway defects may be amenable to treatment with endobronchial stents. In addition, commercially available stents are often limited in their ability to conform to various airway anatomical variations. We describe a case in which we customized a commercially available silicone Y-stent to support a patient with a large post-pneumonectomy BPF.
Case presentation
A 65-year-old gentleman with a past medical history of Sjogren’s disease and chronic kidney disease presented with persistent Mycobacterium szulgai infection and Aspergillus cavitary pneumonias refractory to antibiotic therapy. He underwent a left-sided pneumonectomy given the progression of infection despite optimal medical management (Figure 1). His immediate post-surgical course was uneventful, and he was discharged to pulmonary rehabilitation on post-operative day 10.
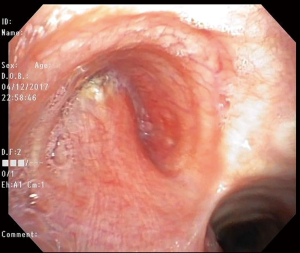
Eleven weeks after surgery, the patient noted increasing dyspnea and a productive cough. A chest CT scan revealed a new left hydropneumothorax with diffuse nodular opacities scattered on the right lung. A diagnostic flexible bronchoscopy showed a large left-sided BPF involving greater than half of the bronchial stump (Figure 2). Direct communication with the pleural cavity was evident with purulent secretions coming from the BPF that were soiling the contralateral airways.
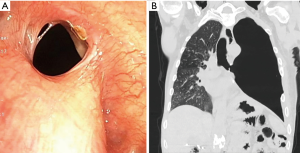
A chest tube was placed and broad-spectrum antibiotic therapy was initiated. Given his complex comorbidities, surgical closure was deferred. Left-sided pleural space debridement and washing followed by Kerlix packing and Eloesser flap creation was performed. To protect the right lung during the procedure, an 18 mm × 14 mm × 14 mm Y-shaped endobronchial Dumon® stent (Novatech SA, La Ciotat, France), customized by stapling the left limb with a mechanical Endo GIATM stapler (Medtronic, Minneapolis, MN, USA) at 10 mm from the carina (Figure 3) was placed bronchoscopically. Size determination of the silicone Y-stent and the length of the left limb were based on measurements of the airway diameter and distance from the main carina to the BPF, which were obtained by reviewing the CT chest and confirmed bronchoscopically. Unfortunately, due to a persistent air leak with positive pressure ventilation, the stent was removed and right mainstem intubation was performed.
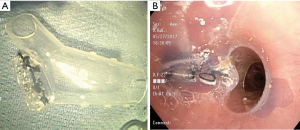
Due to the patient’s ongoing respiratory decline and persistent infection of the contralateral lung, a second attempt at endobronchial stenting was undertaken with the goal of sealing off the left limb of the stent more effectively. A second 18 mm × 14 mm × 14 mm Y-shaped Dumon® stent (Novatech) was cut at the left limb 20 mm from the distal end and was invaginated, preserving the stent’s circular contour. Circumferential mattress sutures (silk size 0) were used to tie in the distal left limb (Figure 4), leaving a 10 mm bronchial limb as measured during bronchoscopy. DermabondTM (2-Octyl cyanoacrylate) (Ethicon Inc., Somerville, NJ, USA) was instilled within the stent to seal any remaining spaces along the suture line. The patient required tracheostomy placement due to prolonged mechanical ventilation, and he was discharged after 3 weeks.
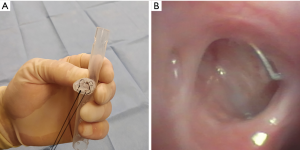
Repeat bronchoscopy with an airway survey and Y-stent revision was performed three weeks after discharge and demonstrated early signs of stump epithelialization without any evidence of purulent spillage. The stent was washed with saline solution and hydrogen peroxide and replaced. Ten months after initial stent placement significant improvement of patients’ clinical condition was noted, including a twenty-pound weight gain and ability to participate in pulmonary rehabilitation. Bronchoscopic assessment confirmed closure of fistula (Figure 4B) by submerging the bronchial stump on saline solution through the Clagett window. The stent was then removed and the Clagett window was closed surgically.
Discussion
BPFs are a rare complication following pneumonectomy and their outcomes are often poor (2). The first line of treatment is surgical closure with a muscle flap (7) but this may be high risk in patients with a poor health status or active infection. In these cases, various endoscopic alternatives have been proposed to help reduce the pressure gradients between the airways and the pleural cavity while protecting healthy airways from purulent overflow (8). Most of the data available on endoscopic interventions for BPF includes patients who had undergone lobectomies; there is only a small number of post-pneumonectomy patients with BPFs reported. Bronchoscopic treatment varies depending on the size of the BPF; from the use of mechanical abrasion using a flexible wire brush for small (≤2 mm) fistulas to the insertion of silicone spigots in large (>6 mm) fistulas (2). Other strategies reported in the literature include placing silicone endobronchial cufflinks (9), covered esophageal stents (10) and devices designed to close cardiac septal defects (11). The application of tissue glues has also been considered as a feasible alternative (12).
While these strategies may be acceptable for distal BPFs, the treatment of proximal BPFs requires special consideration. There is a risk of contralateral airway obstruction if an airway device meant to occlude the BPF becomes dislodged. Thus, stents are an attractive intervention for large proximal BPFs.
Amaral and Feijó reported the use of a silicone Y-shaped endobronchial stent with a mechanically-stapled distal limb to support the closure of a BPF (13). Similarly, Jindal and Agarwal proposed the manual creation of an endobronchial spigot by folding a silicone stent (14). Freitag and colleagues recently published a review that describes the use of customized 3D-printed tracheobronchial stents. Unfortunately, costs associated with these stents are often higher, and may take several days to become available as they are produced on-demand (15). The use of nitinol-mesh self-expanding and, in some cases customized stents, has been described in several case series (4,5).
Manual modification of endobronchial stents is an affordable alternative that is readily available. This approach may be particularly useful to temporarily contain complicated intrathoracic infections in patients with BPFs and prevent soiling of healthy lung segments, while dehiscent tissue re-epithelializes. A manual modification that preserves the stent’s tubular structure and circular contour is an inexpensive and instantaneous alternative to achieve this, potentially favoring the transition to spontaneous ventilation. Although modified silicone stents may potentially reduce the amount of air leak through the BPF, this is not their main objective. In this case, the main goal for the modification of the silicone stent was to prevent spillage of the purulent left-sided pleural cavity towards the right-sided airways.
Conclusions
Different stent designs to support BPF epithelialization have been proven useful, but these may not always be readily available. For BPF cases which demand immediate and cost-effective sealing, the use of customizable silicone stents is a valuable alternative treatment that should be considered.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent has been obtained from the patient’s healthcare proxy (daughter) for publication of this case report and any accompanying images.
References
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [Crossref] [PubMed]
- Cardillo G, Carbone L, Carleo F, et al. The Rationale for Treatment of Postresectional Bronchopleural Fistula: Analysis of 52 Patients. Ann Thorac Surg 2015;100:251-7. [Crossref] [PubMed]
- Mao R, Ying PQ, Xie D, et al. Conservative management of empyema-complicated post-lobectomy bronchopleural fistulas: experience of consecutive 13 cases in 9 years. J Thorac Dis 2016;8:1577-86. [Crossref] [PubMed]
- Andreetti C, D'Andrilli A, Ibrahim M, et al. Effective treatment of post-pneumonectomy bronchopleural fistula by conical fully covered self-expandable stent. Interact Cardiovasc Thorac Surg 2012;14:420-3. [Crossref] [PubMed]
- Cao M, Zhu Q, Wang W, et al. Clinical Application of Fully Covered Self-Expandable Metal Stents in the Treatment of Bronchial Fistula. Thorac Cardiovasc Surg 2016;64:533-9. [Crossref] [PubMed]
- Dutau H, Breen DP, Gomez C, et al. The integrated place of tracheobronchial stents in the multidisciplinary management of large post-pneumonectomy fistulas: our experience using a novel customised conical self-expandable metallic stent. Eur J Cardiothorac Surg 2011;39:185-9. [Crossref] [PubMed]
- Mehta RM, Singla A, Bhat RS, et al. An Innovative Solution for Prolonged Air Leaks: The Customized Endobronchial Silicone Blocker. J Bronchology Interv Pulmonol 2018;25:111-7. [PubMed]
- Fuso L, Varone F, Nachira D, et al. Incidence and Management of Post-Lobectomy and Pneumonectomy Bronchopleural Fistula. Lung 2016;194:299-305. [Crossref] [PubMed]
- Colt HG, Murgu SD. Closure of pneumonectomy stump fistula using custom Y and cuff-link-shaped silicone prostheses. Ann Thorac Cardiovasc Surg 2009;15:339-42. [PubMed]
- Jones NC, Kirk AJ, Edwards RD. Bronchopleural fistula treated with a covered wallstent. Ann Thorac Surg 2006;81:364-6. [Crossref] [PubMed]
- Klotz LV, Gesierich W, Schott-Hildebrand S, et al. Endobronchial closure of bronchopleural fistula using Amplatzer device. J Thorac Dis 2015;7:1478-82. [PubMed]
- Hamid UI, Jones JM. Closure of a bronchopleural fistula using glue. Interact Cardiovasc Thorac Surg 2011;13:117-8. [Crossref] [PubMed]
- Amaral B, Feijó S. Fistula of the Stump: A Novel Approach With a "Stapled" Stent. J Bronchology Interv Pulmonol 2015;22:365-6. [Crossref] [PubMed]
- Jindal A, Agarwal R. Novel treatment of a persistent bronchopleural fistula using a customized spigot. J Bronchology Interv Pulmonol 2014;21:173-6. [Crossref] [PubMed]
- Freitag L, Gördes M, Zarogoulidis P, et al. Towards Individualized Tracheobronchial Stents: Technical, Practical and Legal Considerations. Respiration 2017;94:442-56. [Crossref] [PubMed]


