Spread through air spaces predicts a worse survival in patients with stage I adenocarcinomas >2 cm after radical lobectomy
Introduction
Lung cancer has become the first malignant tumor and leading cancer-related death causes due to the air pollution caused by industry process and increase of smoking and aged population in China (1). A considerable progress in the screening of lung cancer has led a tremendous increase of early detection of patients with stage I lung cancer, surgery is still the standard treatment for them (2). However, about 20.00% of stage I patients undergone radical resection still have local recurrence or distant metastasis (3). Though much effort has been reported in searching for biomarkers that aid in predicting the prognosis of patients with early lung adenocarcinomas (4,5), tumor size is still an irreplaceable prognostic factor, especially in the newly published 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual lung cancer staging system, which emphasizes much more on each centimeter increase in tumor size (6,7).
In addition to the tumor size, pathological findings of tumor spread through air spaces (STAS) has also been reported as an invasive pattern of lung adenocarcinoma in the newest WHO guidelines (8), which is defined as single cancer cells, micropapillary clusters, or solid nests that are observed within air spaces in the surrounding lung parenchyma beyond the edge of the tumor (9). Several studies demonstrated that STAS was a convincing explanation for recurrence of the patients with early lung cancers who underwent limited resections. However, inconsistency was found in patients undertaken lobectomy. Some authors redeemed that STAS was a worse survival parameter in lobectomy, while others didn’t think so (10-12). Thus, we performed a survival analysis using a single center cohort of patients with radically resected lung adenocarcinoma ≤4 cm (8th AJCC pStage I) to determine whether STAS could further stratify the survival within different tumor size subsets.
Methods
Case selection
This retrospective study was approved by National Cancer Center’s Institutional Review Board (No. NCC2014ST-07). Pathologic stage determination was primarily based on the 7th edition of the American Joint Committee on Cancer Staging Manual (13) and reclassified according to the 8th edition staging manual (6,7). All pathologically diagnosed pulmonary adenocarcinoma with radical surgical resection were consecutively collected from the Department of Pathology of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences from January 2009 to September 2011. All patients with lung adenocarcinomas diagnosed as pathological stage I disease underwent radical resection were reviewed. Inclusive criteria were limited to: (I) tumor size ≤4 cm; (II) solitary nodule; (III) no neoadjuvant therapy; (IV) curative-intent lobectomy with sufficient lymph node dissection (≥3 of mediastinal lymph node stations including subcrinal station and a total resected lymph nodes number >6); and (V) precise preoperative evaluation including computed tomographic scans, head magnetic resonance imaging (MRI) and bone scan prior to surgical resection in order to exclude clinical stage II or above. Exclusive criteria were: (I) multiple nodules; (II) clinically reported or suspicious of any lymph node or distant metastasis status, (III) patients who received preoperative radiation/chemotherapy, (IV) patients died of postoperative complications, (V) patients had other malignancy except for lung cancer, (VI) patients without sufficient following-up information, and (VII) no available tumor slides for review. According to these criteria, a total of 242 patients were eventually selected from the database. All recurrences were confirmed by clinical and radiological/ pathological assessment. The medical records and database were reviewed for updating patients’ follow-up information till December 30th, 2015.
Histologic evaluation
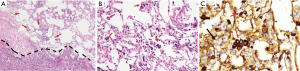
Tumor slides were reviewed by two experienced pathologists (L Yang and PQ Ma) who were blinded to patient clinical outcomes. Elastic staining was done for suspicious visceral invasion cases. Tumor STAS was defined as tumor cells within air spaces in the lung parenchyma beyond the edge of the main tumor, which was composed of three morphological patterns (micropapillary structures, solid nests or tumor islands, and single cells) within air spaces beyond the second alveolar layer from the edge of main tumor. Tumor cells of STAS were distinguished from alveolar macrophages or artifacts according to Kadota et al. (9) and immunostaining of AE1/AE3 in some of the difficult cases.
According to the histological classification of lung cancer published by World Health Organization published in 2015 (8), the percentage of each histological pattern (lepidic, acinar, papillary, solid, and micropapillary) was recorded in 5.00% increments and tumors were classified by their predominant pattern. In addition to STAS and subtype of histological classification, other pathological parameters were recorded, such as presence of visceral pleural involvement, lymph-vascular invasion, bronchial invasion, neural invasion, etc. If disagreement occurred, discussion was performed before reaching a consensus between the two pathologists.
Other clinicopathological data were extracted from medical archives, including gender, age, smoking history, family history, tumor size, postoperative treatment, and so on.
Immunohistochemistry and elastin staining
AE1/AE3 immunohistochemistry staining was applied when there was difficultly in distinguishing STAS with macrophages in some cases, using the EnVision Plus detection system (DAKO, Carpinteria, CA, USA) with controls. Also, elastin staining was adopted for confirmation of visceral pleural involvement, staining process was done according to Zhao et al. (14). Totally, there were 38 slides underwent elastin staining and AE1/AE3 immunohistochemistry staining.
Follow-up
All enrolled patients were conducted a follow-up from the date of surgery to December 30th, 2015, with a follow-up interval of 49 to 78 months. Disease-free survival (DFS) period was calculated from the date of surgery to the date of surgical treatment failure (local recurrence or distant metastasis confirmed by image scan or pathological biopsy). Overall survival (OS) period was defined from the date of surgery to death or last follow-up (due to December, 30th, 2015).
Statistical analysis
Chi-square test or Fisher’s exact test were used to compare frequency of clinicopathological characters between STAS (+) and STAS (−) groups. DFS and OS curves were estimated using the Kaplan-Meier method. Log rank test, Cox univariate and multivariate proportional hazards regression were used for adjusting associated confounding factors. The statistical analyses were conducted using SPSS version 23.0 software (SPSS, Chicago, IL, USA), and statistical significance was set as P<0.05.
Results
Patient characteristics and their correlation with STAS status
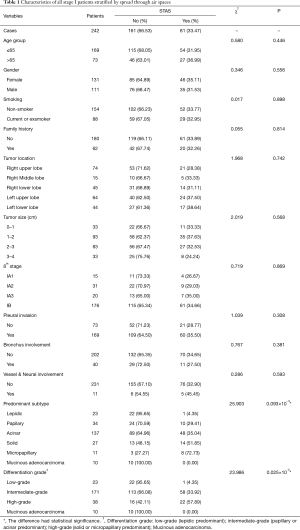
All included 242 cases were listed in Table 1, stratified by STAS, with 47 recurrence or metastasis and 25 death cases. The mean age of all enrolled patients was 58.73 yrs, with a female/male ratio of 1.18 (131/111). STAS morphology and AE1/AE3 immunohistochemical staining was also showed in the Figure 1. Clinical features were compared between STAS (+) and STAS (−) groups, no significant difference was found in such parameters as age, gender, smoking, family history, tumor location, tumor size with 1cm increment, 8thpStage (P>0.05, seen in Table 1). Further analysis stratified by 8th AJCC staging system, there’s no significant difference between STAS (+) and STAS (−) patients in OS and PFS (P>0.05). Neither significant findings were observed on pleural invasion, bronchus & vessel & neural involvement in STAS (+) and STAS (−) groups (P>0.05, seen in Table 1).
Full table
Correlation of STAS with predominant histological pattern and differentiation grade
All enrolled cases were reviewed according to the 2015 WHO histological classification and the predominant histological subtypes were recorded, including 23 cases of lepidic subtype, 34 papillary, 137 acinar, 27 solid, 11 micropapillary, and 10 mucinous adenocarcinomas. STAS was observed in 81 cases of this series (33.47%) which was the lowest in lepidic subtype (4.35%, 1/23), 29.41% (10/34) in papillary subtype, 35.04% (48/137) in acinar subtype, 51.85% (14/27) in solid predominant subtype, and the highest in micropapillary predominant ones (72.73%, 8/11). There was a significant difference in STAS positive status among different predominant subtypes (χ2=25.903, P=0.093×10−3) (Table 1).
Differentiation grade was also recorded and the correlation with STAS positive status was analyzed. In short, highly differentiated subtype was defined as lepidic (9.50%, 23/242), moderately differentiated subtypes included papillary and acinar predominant (70.67%, 171/242), and poorly differentiated subtype refers to micropapillary or solid (15.70%, 38/242). The poorly differentiated subtype showed the highest STAS positive rate (χ2=23.986, P=0.025×10−3) (seen in Table 1).
Tumor STAS affected survival in adenocarcinoma stratified by tumor size
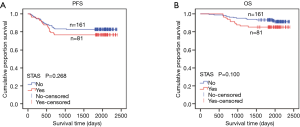
All enrolled cases were classified into two groups according to tumor size: 0–2 cm (0< tumors ≤2.0 cm) or 2–4 cm (2< tumors ≤4 cm) .Univariate analysis of all enrolled patients showed that tumor size (0< tumors ≤2.0 cm vs. 2< tumors ≤4 cm) was an independent parameter both for PFS (P=0.016×10−3, 95% CI, 0.159–0.501) and OS (P=0.054×10−7, 95% CI, 0.061–0.294). STAS positive rate was 36.51% (46/126) in the group with tumor size ≤2 cm and 30.17% (35/116) in the group with tumor size of 2–4 cm (2< tumors ≤4 cm). Among all stage I patients, a worse survival trend of PFS and OS was found in the STAS (+) group than in the STAS (−) group, but without statistical significance (PFS, P=0.268; OS, P=0.100) (Figure 2, Table S1).
Full table
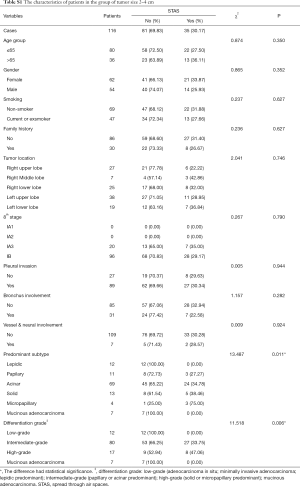
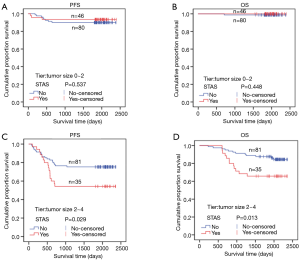
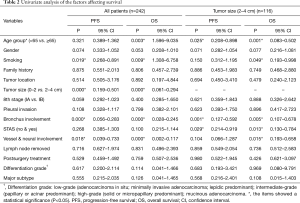
STAS status showed different survival significance in the two groups stratified by tumor size as 0–2 cm (0< tumors ≤2 cm vs. 2–4 cm (2< tumors ≤4 cm). Figure 3A,B indicated no significant difference for STAS (+) or STAS (−) status in PFS (P=0.537) and OS (P=0.448) in patients with tumors ≤2 cm. However, STAS significantly affected PFS (P=0.029, 95% CI, 0.214–0.919) and OS (P=0.013, 95% CI, 0.130–0.784) in patients with tumors between 2.0 to 4.0 cm (Figure 3C,D). Multivariate analysis showed STAS (+) status was an independent factor affecting both PFS (P=0.004, 95% CI, 0.177–0.723) and OS (P=0.002, 95% CI, 0.088–0.563) in patients with tumors within 2–4 cm (2< tumors ≤4 cm). Detailed results are presented in Tables 2,3. Further analysis for correlation of STAS status with the time to recurrence and metastasis or death showed no significant difference both in overall patients (PFS, P=0.779; OS, P=0.920) and group 2–4 cm (PFS, P=0.727; OS, P=0.082).
Full table
Full table
Discussion
In this series, STAS was found in 81 (33.47%) among 242 patients who underwent radical lobectomy and adequate hilar and mediastinal lymphadenectomy. The presence of STAS was significantly associated with pathologically invasive major subtypes and a poor differentiation such as micropapillary or solid predominant subtypes, and STAS (+) patients showed a significant lower DFS and OS in the patients with a larger tumor size of 2–4 cm (2< tumors ≤4 cm). Our findings were consistent with the previous reports (9-11), which indicated that a subset of stage I adenocarcinoma with STAS might need more intensive follow-up or adjuvant treatment after surgical resection.
The TNM staging system has been the gold standard parameter for predicting survival and guide to treatment strategy for primary lung cancer (6). The newly revised 8th AJCC lung cancer staging system stratified tumor size with 1cm increment in early lung cancers unless pleural involvement, which is directly classified into T2a irrespective of tumor size in which the precisely sub-grouping may be more valuable in early-stage tumors for making a decision whether sub lobectomy or lobectomy is adequate (15). For stage I lung cancer, TNM staging seems not to be a strong predictor for recurrence or long-term survival (16). Instead, tumor size was found in the current study to be an independent predictor for both PFS and OS.
In recent years, STAS attracted more attention since it was first reported as a specific invasion pattern for lung adenocarcinoma in 2015 (8), and was presented as a risk factor of disease recurrence according to types of surgical procedures (lobectomy or limited resection) and location of recurrence (locoregional or distant) (9). Later on, a growing number of studies have reported their findings and supported the preliminary conclusions as a negative predictor of survival for patients with lung adenocarcinomas (9,11). In this study, we found that STAS was more frequently seen in the highly invasive adenocarcinoma such as solid and micropapillary adenocarcinoma, and less frequently in lepidic predominant adenocarcinomas. This is consistent with the previous studies (12). In the initial report, STAS was reported as a risk factor in the prognosis of patients who underwent limited resection, but not those who underwent radical lobectomy (9). Another study from Japan also indicated that STAS was significantly associated with a lower OS in the limited resection group, while no significant correlation was found between STAS and the OS in the lobectomy groups (12). In the current study, all enrolled patients underwent radical lobectomy and standard lymphadenectomy, and no statistically significant difference in the survival was found between STAS (+) and STAS (−) groups in the whole series, which is consistent with the above studies. However, when stratified both by STAS in different pathological stages, our data showed no significant difference, on which we presumed that STAS was not correlated with tumor stage but correlated with tumor subtypes or differentiation grade. Further, when stratified by tumor size with a cut-off 2 cm, we found that STAS in the 2–4 cm (2< tumors ≤4 cm) group was an independent worse survival parameter, which was consistent with the recent study reported by Dai et al. In the study reported by Dai’s, 95.00% of patients underwent lobectomy and the results showed STAS significantly affecting recurrence and survival, especially in tumor size larger than 2 cm (11). Therefore, the significance of STAS in small-sized pulmonary adenocarcinomas treated with radical lobectomy deserves to be further investigated in future prospective studies stratified by tumor size.
It is a logical assumption that tumor size might be a stratifying factor for assessing the significance of STAS on patients’ survival, for tumor size has been reported to be an independent survival factor both for patients underwent curative-intent surgery or radiotherapy or chemotherapy, especially in tumor size less than 3.0 cm (17,18). Thus, we investigated the significance of the combination of STAS and tumor size on the survival in pathological stage I patient. Our result was similar to that reported by Dai et al., in which, STAS statistically stratified the prognosis of patients with adenocarcinomas between 2 to 3 cm (2< tumors ≤3 cm), and similar to that of patients with stage IB adenocarcinoma (11). Therefore, STAS should be considered as a prognostic factor in the early lung adenocarcinomas >2 cm.
Although currently lobectomy with hilar and mediastinal lymph node dissection was still the standard treatment for early-stage lung cancers, for the peripheral small lung adenocarcinomas ≤2 cm, limited resection such as wedge resection or segmentectomy may be comparable to the lobectomy in the outcome, in such case, STAS remains to be a recurrence or metastasis risk factor. Therefore, distinguishing of STAS on frozen diagnostic sections would be beneficial for surgical strategy whether partial lung resection is enough or not (19,20). In our experience, the dilemma of frozen diagnosis for STAS is to distinguish it from macrophages. In the current study, AE1/AE3 immunohistochemistry method was applied to differential diagnosis, and the staining results was proved to be very useful for identifying STAS. As far as we know, this has not been reported in literatures for detecting STAS. A rapid immunohistochemical method (21) of AE1/AE3 for detecting STAS on frozen sections will be valuable for further study, and our preliminary result is promising (data will be shown in next study). Still, AE1/AE3 immunostaining should be cautiously explained when sometimes the exfoliated bronchial epithelia are also stained with AE1/AE3, which may be the shortcoming of AE1/AE3 for STAS identification. In this case, the morphological identification of cell atypia should be considered more seriously.
STAS is not only correlated with an increased recurrence/metastasis rate in some patients with early stage lung adenocarcinomas, but also is found correlated with PET-CT features. In our another study of 121 stage I lung adenocarcinomas (32 of which came from the current cohort), STAS was found positive in 49 cases, and a significant difference of STAS distribution was found among different nodule types (solid vs. non-solid types, P=0.001) and metabolic tumor burden parameters, the standardized uptake value (SUVmax, P=0.020; SUVmean, P=0.002 and SUVpeak, P=0.002) and total lesion glycolysis (TLG, P=0.004) (data to be published). Furthermore, in our another published study, artificial intelligence radiomics was applied in observing the peripheral features of PET-CT scanned non-small cell lung cancer with a hypothesis that non-invasive and invasive tumor boundary may have different morphological patterns, a prediction model named with “shell” was created for predicting distant failure of lung cancer after curative-intent surgery (22), which is a promising work for further study on identifying STAS and other PET/CT features by artificial intelligence for evaluating surgical method before operation and prognosis after curative-intent surgery.
In conclusion, presence of STAS is a significant worse predictor for the patients with lung adenocarcinoma >2 cm underwent radical lobectomy and lymphadenectomy, while it is not significant in patients with tumor size ≤2 cm. Existence of STAS reveals an increased risk of recurrence and/or metastasis for tumors lager than 2 cm in stage I patients. The combination of tumor size and STAS status may be helpful in assessing the extent of surgical resection and follow-up strategy or post surgery treatment.
Acknowledgements
Funding: The study was supported by the PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3332015060), and Beijing Municipal Science & Technology Commission (Research on the application of clinical characteristics in the capital, Z141107002514047).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by National Cancer Center’s Institutional Review Board (No. NCC2014ST-07).
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:504-35. [Crossref] [PubMed]
- Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013;143:1626-34. [Crossref] [PubMed]
- Ren Y, Zhao S, Jiang D, et al. Proteomic biomarkers for lung cancer progression. Biomark Med 2018;12:205-15. [Crossref] [PubMed]
- Zhang J, Shao J, Zhu L, et al. Molecular profiling identifies prognostic markers of stage IA lung adenocarcinoma. Oncotarget 2017;8:74846-55. [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Yang L, Wang SD, Zhou YY, et al. Evaluation of the 7th and 8th editions of the AJCC/UICC staging systems for lung cancer in a large North American cohort. Oncotarget 2017;8:66784-95.
- William DT, Elisabeth B, Allen PB et al. WHO Classification of Tumours of Lung, Pleura, Thymus and Heart, 4th ed. IARC: Lyon 2015.
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma >2 to 3 cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of Spread Through Air Spaces in Resected Pathological Stage I Lung Adenocarcinoma Ann Thorac Surg 2018;105:1655-63. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. p. 253-270
- Zhao LX, Yu KK, Shao JC, et al. Clinical significance of elastic fibers staining in assessing the pleural invasion of lung cancer. J Clin Exp Pathol 2014;30:22-4.
- Malhotra J, Mhango G, Gomez JE, et al. Adjuvant chemotherapy for elderly patients with stage I nonsmall-cell lung cancer 4 cm in size: an SEER-Medicare analysis. Ann Oncol 2015;26:768-73. [Crossref] [PubMed]
- Dai C, Shen J, Ren Y, et al. Choice of surgical procedure for patients with non-small-cell lung cancer <=1 cm or >1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol 2016;34:3175-82. [Crossref] [PubMed]
- Zhang Y, Sun Y, Chen H. Effect of tumor size on prognosis of node-negative lung cancer with sufficient lymph node examination and no disease extension. Onco Targets Ther 2016;9:649-53. [Crossref] [PubMed]
- Ball D, Mitchell A, Giroux D, et al. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol 2013;8:315-21. [Crossref] [PubMed]
- Konaka C, Ikeda N, Hiyoshi T, et al. Peripheral non-small cell lung cancers 2.0 cm or less in diameter: proposed criteria for limited pulmonary resection based upon clinicopathological presentation. Lung Cancer 1998;21:185-91. [Crossref] [PubMed]
- Xiao F, Yu Q, Zhang Z, et al. Novel perspective to evaluate the safety of segmentectomy: clinical significance of lobar and segmental lymph node metastasis in cT1N0M0 lung adenocarcinoma. Eur J Cardiothorac Surg 2018;53:228-34. [Crossref] [PubMed]
- Zhang CX, Guo SJ, Wang Q. Expression of C-MET in thyroid carcinoma detected by rapid immunohistochemical analysis and its clinical significance for predicting lymph node metastases. Chin J Gen Surg 2010;19:471-4.
- Hao H, Zhou Z, Li S, et al. Shell feature: a new radiomics descriptor for predicting distant failure after radiotherapy in non-small cell lung cancer and cervix cancer. Phys Med Biol 2018;63. [Crossref] [PubMed]


