Comparative study of video-assisted thoracic surgery versus open thymectomy for thymoma in one single center
Introduction
Thymoma is one of the most common mediastinal tumors, which accounts for about 47% of anterior mediastinal tumors (1,2). It seems to be benign tumor but recurs and metastasizes easily, which leads to treatment with challenges. As it is well known, thymectomy was considered as the only curative treatment for resectable thymoma patients (3). Excellent 5- and 10-year-survival rates are noted for completely resected early stage-thymomas (4). Moreover, several findings had indicated that the thymus was involved in myasthenia gravis pathogenesis (5) and the most common indication for video-assisted thoracic surgery (VATS) thymectomy was the treatment of myasthenia gravis (6).
In 1992, Landreneau et al. (7) introduced the first thymoma patient with VATS thymectomy and after that, the number of thymoma patients with VATS thymectomy has rapidly increased. However, arguments on this technique always exist. Compared with open thymectomy, the potential superiorities and disadvantages of VATS thymectomy remained controversial. Since 2007, VATS thymectomy has been introduced and conducted in our hospital. From our experience of VATS thymectomy from January 2007 to September 2013, we tried to compare the outcomes of VATS thymectomy to that of open thymectomy including sternotomy and thoracotomy in a single institute retrospectively so as to confirm if patients underwent VATS thymectomy can benefit from this minimal invasive surgery.
Methods
There were 137 consecutive patients who underwent thymectomy diagnosed with early stage thymoma (Masaoka stage I and stage II) in the Cancer Institute & Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College from January 2007 to September 2013. Eight of those thymoma patients who received radiotherapy preoperatively were excluded. Consequently, one hundred and twenty-nine thymoma patients were reviewed retrospectively. We included two groups in our study: one group included 38 patients via VATS thymectomy, and the other group included 91 patients via open thymectomy including either sternotomy or thoracotomy. Computed tomography (CT) was considered the standard diagnostic modality for thymomas (8). All eligible thymoma patients were diagnosed as mediastinal tumor or mass by CT preoperatively and were given clear diagnosis as thymoma by pathology postoperatively, who were restricted to Muller-Hermelink type A, type B (B1/B2/B3) and type AB as well as Massaoka stage I-II. All collected clinical data were analyzed by SPSS 17.0. T-tests were performed for all continuous variables, and Pearson chi-square test or Fisher’s exact test were performed for all categoric variables. P values ≤0.05 were considered significant.
Preoperatively, pulmonary function tests and electrocardiogram were necessary for every patient. None of them received needle biopsies. The symptoms of thymoma patients mainly stated include chest pain, tachypnea and cough. Nonetheless, most of thymoma patients who were in hospital were discovered by health physical examination and did not have any symptoms. Nineteen thymoma patients with myasthenia gravis mainly complained of ptosis or weakness but did not require special treatment.
All patients were managed in the thoracic surgery wards or in the intensive care unit (ICU), postoperatively. The symptoms of twelve thymoma patients with myasthenia gravis were improved or disappeared after thymectomy. We did not remove the chest tubes until the quantity of drainage was less than 200 mL routinely. Obviously, the completeness of resection had to be aimed because it was an important prognostic factor for local control. The whole connective membrane or fat tissue covering the lesion was used to confirm thymoma, which was resected completely by pathologists in our study.
Surgical techniques
VATS thymectomy
The procedures resembled what Jurado et al. (9) described previously. VATS thymectomy was performed from one side. The patients were placed in the supine position with arms extended, and both one shoulder and ipsilateral backside were raised by slipping pads below. Generally, we made two ports for working and one port for viewing in the intercostal spaces in detail according to the location and the size of the lesion by CT. Single-lung ventilation with a double lumen endotracheal tube was necessary for VATS thymectomy. Carbon dioxide insufflation was not required. The dissection was begun with inferior thymic poles mobilized carefully and thoroughly along the pericardium and anteriorly along the retrosternum. The perimediastinal fat tissue and thymus were swept by endoscopic ultrasonic scalpel as well as both pleural spaces had to be opened so that the mass was resected en bloc. All anterior mediastinal tissue was swept cephalad. The innominate vein was located at the junction with the superior vena cava, and dissection continued until the thymic vein was located; this was then doubly clipped. The dissection was completed by dissection along the left pleura which was performed bluntly to avoid injury to the left phrenic nerve. The resected specimen was then placed in an endoscopic pouch. If the mass was too large, we could enlarge one port appropriately or extract the huge mass piece by piece carefully in the endoscopic pouch to avoid potential intrathoracic spreading. One or two drainage tubes were placed unilaterally.
Transsternal thymectomy
As a common approach of open thoracic surgery, transsternal thymectomy was first described by Blalock et al. (10) in 1941, which was adopted by many thoracic surgeons for resectable thymoma. Sternum was incised by an electric motor saw. Bilateral pleural spaces were opened and vein extending from the innominate vein was divided. anteriormediastinal, tissue including the pericardial fat tissue and the removal intact of all poles of thymus was exenterated. Single-lung ventilation was not required. One or two drainage tubes were placed necessarily.
Transthoracic thymectomy
Whether transthoracic thymectomy performed on the left or the right side was based on the location of the thymoma. The patient was positioned on the left or the right lateral supine position that resembled VATS approach, and left or right anterolateral thoracotomy incision was carried out via the 4th intercostal space. Single-lung ventilation was essential as well. Both lobe of thymus were removed by opening bilateral mediastinal pleura. Meanwhile, pericardial fat tissue was swept as much as possible. One or two drainage tubes would be enough in the unilateral thorax.
Results
A number of 129 selected thymectomies were performed in the Cancer Institute & Hospital of Chinese Academy of Medical Sciences from January 2007 to September 2013. Of those, 38 thymoma patients were performed via VATS thymectomy, none of whom transformed to thoracotomy. A number of 91 thymoma patients via open thymectomy including 44 patients via transsternal thymectomy and 47 ones via transthoracic thymectomy. Moreover, 19 thymoma patients with myasthenia gravis, included 5 patients of the ones via VATS thymectomy and 14 patients of the others via open thymectomy respectively. Tumor size was not significantly different between VATS group and open surgery group. Subsequently, it was significantly different between VATS group and sternotomy group (5.4 versus 6.5 cm, P=0.013) as well as between VATS group and thoracotomy group (5.4 versus 7.2 cm, P<0.001). The rest of the data analysed showed that gender, age, BMI, pathological classification, staging, percentage of patients with myasthenia gravis, percentage of patients with complications including hypertension, diabetes mellitus and coronary heart disease as well as percent of main symptoms including tachypnea and cough between VATS thymectomy and open thymectomy were not significantly different. Percentage of chest pain was significantly different between VATS thymectomy and open thymectomy (9 versus 23, P=0.036), taking a selection bias into consideration. Relevant subject characteristics are summarized in Table 1.
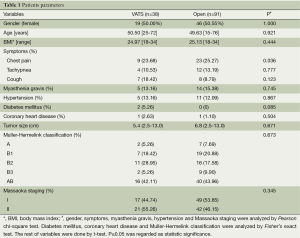
Full table
There was no death or morbidity due to the surgical procedures perioperatively. The ICU length of stay (LOS), operation time, entire resection ratio, and the number of chest tubes were not significantly different in two groups. The postoperative hospital LOS of VATS thymectomy was shorter than that of open thymectomy (5.26 versus 8.32 days, P<0.001). The blood loss of VATS thymectomy was less than open thymectomy (114.74 versus 194.51 mL, P=0.002). Postoperatively, the quantity of chest tubes output in VATS group was less than that in open thymectomy group (617.86 versus 850.08 mL, P=0.007) and duration of drainage in VATS group was shorter than that in open thymectomy group (3.87 versus 5.22 days, P<0.001). Furthermore, compared with patients through transsternal approach, the ones who underwent VATS thymectomy had shorter postoperative hospital LOS (5.26 versus 9.16 days, P<0.001), less operation time (127.63 versus 155.46 min, P=0.024), less blood loss (114.74 versus 214.77 mL, P<0.001), smaller number of chest tubes (1.11 versus 1.41, P=0,001), less quantity of chest tubes output (617.68 versus 806.48 mL, P=0.026) and shorter duration of drainage (3.87 versus 5.68 days, P<0.001). In addition, patients in VATS group had longer operation time (127.84 versus 95.64 min, P=0.002) than that of patients via transthoracic thymectomy while shorter postoperative hospital LOS (5.26 versus 7.53 days, P<0.001), less quantity of chest tubes output (619.68 versus 890.89 mL, P=0.022), shorter duration of drainage (3.78 versus 4.79 days, P=0.009) remained in VATS group. Relevant results are indicated in Tables 2-4.

Full table
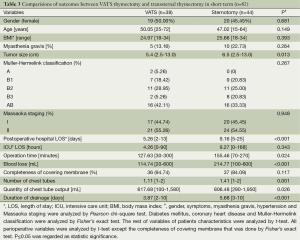
Full table
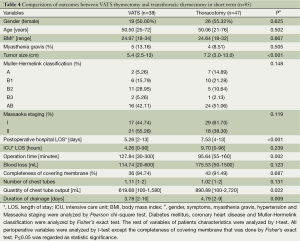
Full table
Discussion
Over the past few decades, advances in techniques have extended the application of VATS approach in thoracic surgery, especially thymectomy. In the past, many reports of VATS thymectomy were small-size retrospective researches, with limitation inevitably. In spite of controversies still existing in long-term curative effect compared with open thymectomy, VATS thymetomy is safe and feasible, and yet had short-term benefits in certain aspects. According to the results above, the gender distribution of thymoma is approximately equal, although it was reported that thymoma was slightly more common in women in older age (11). VATS thymectomy had reached shorter postoperative hospital LOS, less blood loss, less chest output and shorter duration of drainage. These results supported Liu et al. (12) and Pennathur et al. (13) report previously. We also made further contrast between VATS thymectomy and transsternal thymectomy or transthoracic thymectomy respectively. The outcomes indicated that patients who underwent VATS thymectomy had shorter postoperative hospital LOS, less operation time, less blood loss, less quantity of drainage, smaller number of chest tubes and shorter duration of drainage than that of the ones via transsternal approach. Certain results were similar to Meyer’s (14). Patients in VATS group had longer operation time, but shorter postoperative hospital LOS, less quantity of drainage, and shorter duration of drainage than that of patients via thoracotomy. As the heavy wound led to postoperative pain more seriously in the open group, especially by transsternal approach, more postoperative care were required by patients and it delayed their return to normal daily life and work, compared to VATS group. Less incisions, clearer visual field and more accurate dissection, which lead to less blood loss during VATS thymectomy could contribute to recovery and postoperative hospital LOS decreased to less than one week. Without the activity restrictions associated with open thymectomy, patients via VATS approach would return the productive life sooner.
We compared VATS thymectomy with both sternotomy and transthoracic thymectomy comprehensively, especially interiorly. In our 129 thymectomies, there was no death or morbidity due to the surgical procedures perioperatively. Previous reports showed that most of serious complications including vascular injury, chylothorax, nervus phrenicus damage, long-term air leakage and diaphragm injury mainly happened during VATS thymectomy processs. The overall morbidity was about 5.1-10% (3,15-20). Actually, these problems happened during open thymectomy process as well.
Although Blalok et al. (10) had reported in 1941 that thymectomy should be widely managed in treatment of myasthenia gravis, debates remained on the role of surgery in the treatment of myasthenia gravis. The conditions of thymoma patients with myasthenia gravis in our study were not severe preoperatively, and symptoms of 19 thymoma patients with myasthenia gravis had been improved or disappeared after thymectomy. The remission rate of myasthenia gravis was 100% in both VATS group and open surgery group. Gronseth et al. (21) summarized relevant papers and made a conclusion that the curative effect of thymectomy was better than chemotherapy for myasthenia gravis, and the overall remission rate was 70%. Zahid et al. (22) supported that surgical management of MG was becoming increasingly recognised as an effective treatment option. Combined with our results, we believed that thymectomy was an effective therapy for myasthenia gravis patients, especially through VATS approach.
For VATS thymectomy, one major concern is that whether the completeness of resection are comparable similar to open thymectomy. From our experience, we mobilized the poles of thymus and eliminate peri-mediastinal fat as much as possible while achieving a complete removal of the thymic mass. Unilateral VATS approach may certainly limit our ability to resect contralateral tissue accurately. In fact, 36 thymic masses resected (accounting for 95%) in VATS thymectomy group confirmed by pathology had complete covering membrane that was applied to prove that thymus was entirely removed. Based on our results, it had been comparable to 80 open thymectomies (accounting for 88%). The size of the thymic mass is another concern. In our study, tumor size was not significantly different between VATS group and open surgery group. However, we found that, compared to sternotomy group and thoracotomy group respectively, tumor size was smaller in the VATS thymectomy group. Youssef et al. (23) had recommended that less than 3 cm thymoma was appropriate for VATS thymectomy approach. Nonetheless, two thymectomies for thymoma with maximal length-diameter about 13 cm were completed through VATS approach while the similar maximal size of tumor existing in open thymectomy group in our hospital. Therefore, we considered the size of thymoma was not an absolute restriction for VATS thymectomy.
The limitations of the present study should not be neglected. First of all, the number of open group was much larger than that of VATS group, so the selection bias was inevitable. Clinical variables were analyzed in the perioperative period in only one institute, which may lead to inaccurate results. So far, multicentric comparative studies with large sample size for thymoma patients between VATS and open thymectomy groups in a long-term have not been reported. In addition, the follow-up recurrence rate after thymectomy, which was an important assessment for VATS approach, was not found in our study. Cheng et al. (15) showed that 44 thymoma patients who received VATS thymectomy, and followed up for 39.6 months, had no recurrence. Augustin et al. (16) also reported nine thymoma patients via VATS thymectomy did not have recurrence, followed up for 25 months. Hopefully, the shortage of our study may inspire us to do further research.
Conclusions
VATS thymectomy is a safe and practicable treatment for early-stage thymoma patients (Massaoka staging I-II) without evident invasion, which has shorter length of hospital stay, reduced blood loss and less restrictions to activities, hence patients will recover sooner. Eagerly, long-term randomized controlled trials in the multicenter are expected to carry out.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chen JL, Weisbrod GL, Herman SJ. Computed tomography and pathologic correlations of thymic lesions. J Thorac Imaging 1988;3:61-5. [PubMed]
- Qu YJ, Liu GB, Shi HS, et al. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol 2013;20:66-72. [PubMed]
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J 2013;126:2186-91. [PubMed]
- Tomulescu V, Popescu I. Unilateral extended thoracoscopic thymectomy for nontumoral myasthenia gravis--a new standard. Semin Thorac Cardiovasc Surg 2012;24:115-22. [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [PubMed]
- Tomaszek S, Wigle DA, Keshavjee S, et al. Thymomas: review of current clinical practice. Ann Thorac Surg 2009;87:1973-80. [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81. [PubMed]
- Blalock A, McGehee AH, Ford FR. The treatment of myasthenia gravis by removal of the thymus. JAMA 1941;18:1529-33.
- Detterbeck FC. Evaluation and treatment of stage I and II thymoma. J Thorac Oncol 2010;5:S318-22. [PubMed]
- Liu TJ, Lin MW, Hsieh MS, et al. Video-assisted thoracoscopic surgical thymectomy to treat early thymoma: a comparison with the conventional transsternal approach. Ann Surg Oncol 2014;21:322-8. [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg 2011;141:694-701. [PubMed]
- Meyer DM, Herbert MA, Sobhani NC, et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann Thorac Surg 2009;87:385-90. [PubMed]
- Cheng YJ, Hsu JS, Kao EL. Characteristics of thymoma successfully resected by videothoracoscopic surgery. Surg Today 2007;37:192-6. [PubMed]
- Augustin F, Schmid T, Sieb M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann Thorac Surg 2008;85:S768-71. [PubMed]
- Roviaro G, Rebuffat C, Varoli F, et al. Videothoracoscopic excision of mediastinal masses: indications and technique. Ann Thorac Surg 1994;58:1679-83; discussion 1683-4.
- Chetty GK, Khan OA, Onyeaka CV, et al. Experience with video-assisted surgery for suspected mediastinal tumours. Eur J Surg Oncol 2004;30:776-80. [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65. [PubMed]
- Cirino LM, Milanez de Campos JR, Fernandez A, et al. Diagnosis and treatment of mediastinal tumors by thoracoscopy. Chest 2000;117:1787-92. [PubMed]
- Gronseth GS, Barohn RJ. Practice parameter: thymectomy for autoimmune myasthenia gravis (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:7-15. [PubMed]
- Zahid I, Sharif S, Routledge T, et al. Video-assisted thoracoscopic surgery or transsternal thymectomy in the treatment of myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:40-6. [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [PubMed]


