Surgical management of non-mycobacterial fungal infections
Introduction
Fungi are ubiquitous in the environment and infections caused by these organisms are often cleared successfully without intervention in immunocompetent hosts. Immunocompromised patients are at increased risk of fungal opportunistic infection and more likely to have severe illness or develop chronic disease. This patient population is increasing due to higher number of transplantations, use of immunosuppressive medications in non-transplant patients, and neutropenia caused by chemotherapeutic agents. Many fungal organisms have been recovered from respiratory infections, but only a small number of those recovered are consistently implicated as pathogenic (Table 1) (1). Antifungal therapy remains the mainstay of primary pulmonary fungal infection treatment. However, surgery is an important adjunct therapy for invasive infections, resistant infections, mycetoma, cavitary lesions, and empyema. This article will review the pulmonary manifestations of surgically treated non-mycobacterial fungal infections, commonly employed surgical techniques, and discuss post-operative morbidity and mortality rates.

Full table
Aspergillus
There are over 200 recognized species of Aspergilli, although only a few are thought to be pathogenic (2). Aspergillus infections are the number one cause of mortality from invasive fungal disease within the United States. These fungi are pervasive in the environment and typically exist as septate hyphae when present in human tissue. At risk populations include individuals with impaired immunity or patients with chronically diseased lungs. Chronic pulmonary disease may lead to architectural distortion of the pulmonary parenchyma such as bronchiectasis, bullae, cysts, or cavities. Fungal disease can flourish in these anatomical regions of weakness in the lung. The various respiratory diseases caused by Aspergillus range from allergic to infectious in nature (Table 2). Patients initially diagnosed with one form of Aspergillus infection can develop a more invasive or severe form of infection over time (3).
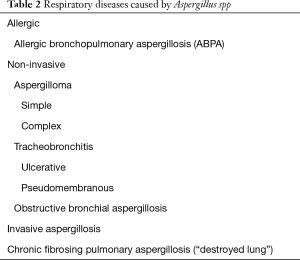
Full table
Allergic bronchopulmonary aspergillosis (ABPA) is an immunological pulmonary disorder caused by hypersensitivity to Aspergillus fumigatus, manifesting with poorly controlled asthma, recurrent pulmonary infiltrates and bronchiectasis (Figure 1) (4). Invasive aspergillosis is a severe form of disease with high risk for parenchymal destruction, invasion into the pulmonary circulation, and disseminated disease. The non-invasive forms of Aspergillus infection include those limited to the airway and aspergilloma.
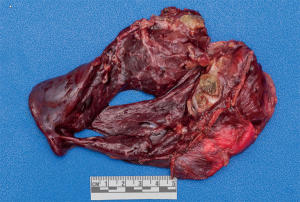
A mycetoma is a mass of fungi colonizing a pre-existing area of weakness within the lung such as a cyst or cavity (2,3,5). An aspergilloma is a mycetoma caused by an Aspergillus species. Simple aspergillomas are stable solitary lesions in a thin walled cavity with adjacent normal lung parenchyma and no pleural involvement (5). Complex lesions are characterized by thick walled cavities and destruction of surrounding lung parenchyma and pleura (Figure 2) (5). The clinical presentation of aspergilloma is widely variable and usually related to the complexity of the disease. Patients may be asymptomatic with an incidental finding or may present in extremis with massive hemoptysis. Common symptoms of aspergilloma include productive cough, weight loss, fatigue, hemoptysis, chest pain, and fever.
Hemoptysis is a potentially life threatening complication of aspergilloma and can be the first presenting symptom of the disease. The incidence of hemoptysis in Aspergilloma ranges from 54–87.5%, with massive hemoptysis rates of 5–10% (6,7). Size, aspergilloma complexity, or prior minor hemoptysis events can predict the patients who develop massive hemoptysis (8). The mechanism for bleeding is erosion of the infected cavity into a nearby artery, most commonly a bronchial artery. A retrospective review of interventional procedures performed on 25 pulmonary aspergilloma patients with massive hemoptysis (see article on Massive Hemoptysis in this issue) documented various sources of arterial bleeding. Of the 68 identified bleeding arteries there were 36 bronchial arteries (52.94%), 15 intercostal arteries (22.06%), 9 internal thoracic arteries (16.17%), 5 inferior phrenic arteries (7.35%), and 3 pulmonary arterial branches (4.41%) (9).
Diagnosis of aspergilloma may be challenging. Isolated sputum culture is negative in 50% of cases. Diagnostic yield is increased with bronchoscopy with bronchoalveolar lavage (BAL), Aspergillus antibodies, immunoassays, and quantitative polymerase chain reaction assays (10). Computed tomography (CT) of the chest can identify the anatomic location of the mycetoma, as well as the relationship to surrounding hilar, vascular, or bronchial structures.
The role of medical therapy varies with the type of pulmonary aspergillus infection. Intravenous or oral antifungal therapy is important for treatment of invasive aspergillus and chronic necrotizing pulmonary aspergillosis. Corticosteroids are utilized in cases of ABPA and hypersensitivity pneumonitis. Currently, there are no antifungal recommendations for primary treatment of aspergilloma. Surgical resection is the treatment of choice (11).
Operative planning depends on aspergilloma size, location, overall disease state of the affected lung, and patient performance status. Lung preservation is important, particularly in patients with limited pulmonary reserve. However, full resection of the aspergilloma is essential to prevent recurrent infection. Pre-operative workup in stable patients includes pulmonary function evaluation, lung perfusion scanning, optimization of nutrition, and performance status assessment. Wedge resections or non-anatomic lung resections are reserved for small peripheral simple aspergillomas (3,12). More frequently a segmentectomy or lobectomy is required to ensure fungal eradication. Operative approaches include both video assisted thoracoscopic surgery (VATS) as well as open thoracotomy. A higher percentage of aspergilloma cases in many studies are performed via thoracotomy. Dense adhesions, scarring, and indurated hilar structures make minimally invasive approaches high risk or contraindicated (3). However Chen et al. demonstrated increased use of VATS in patients with both simple and complex aspergilloma with reduced complication rate in the VATS group and no difference in post-operative mortality between the two groups (13). A pneumonectomy may be required for central lesions or a lung which is no longer functioning due to overwhelming destruction from disease. Morbidity and mortality from a pneumonectomy for fungal disease is high and should only be considered in optimal candidates.
Patients who are not candidates for pulmonary resection can be treated with cavernostomy. A cavernostomy creates an opening into the fungal cavity through a pneumonotomy to allow for drainage and evacuation of fungal debris. The cavern is left open and packed with gauze which is changed as needed and eventually removed. The cavernostomy is left to close spontaneously, or can be surgically closed with placement of an extra thoracic muscle into the residual space or thoracoplasty once the patient recovers from acute illness (14,15). This procedure can temporize patients who are not good operative candidates, but the overall results are inferior to pulmonary resection due to high rates of recurrent Aspergillus infection (14).
Aspergillus empyema can result from primary pleural infection, perforation of an aspergilloma into the pleural space, or breakdown of a bronchial stump after surgical resection. Surgical management for primary empyema includes complete pulmonary decortication with removal of the perforated or associated aspergilloma. This is a tedious operation, particularly in patients with marginal lung function, and care must be taken to perform a complete decortication to prevent recurrent infection. For poor surgical candidates or unstable patients window thoracostomy for open drainage of the infected space is an alternative strategy (15). A window thoracostomy is also the treatment of choice in cases of bronchial stump breakdown after a pneumonectomy resulting in a post-pneumonectomy empyema. Once the pleural and pulmonary infections have been eradicated with adequate drainage and wound care, the residual pleural cavity can be filled with muscle transposition or thoracoplasty (3,15).
Common surgical complications of pulmonary aspergillosis infections are listed in Table 3 (3). Baseline patient characteristics, diseased lung states, dense adhesions, obliteration of normal tissue planes, poor nutritional status, and wound healing issues all contribute to post-operative morbidity and mortality with historical rates as high as 60% (5). Although these are challenging operations, morbidity and mortality rates in surgically treated aspergilloma patients are improving. Contemporary studies report operative mortality rates of 0.9–3.3% and post-operative morbidity rates of 23.6–33.3% (16,17).
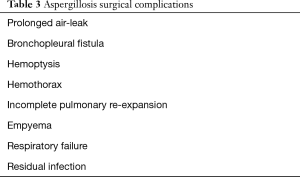
Full table
Coccidioidomycosis
Coccidioidomycosis is caused by Coccidioides immitis and Coccidioides posadasii, dimorphic fungi which grow in arid, desert conditions within the Western hemisphere. Arizona, New Mexico, western Texas, and the central valley of California are endemic regions in the United States. The incidence of coccidioidomycosis is increasing, particularly in light of population increases within the endemic area and frequent domestic and international travel to this area. The estimated hospital cost for patients with coccidioidomycosis was $2 billion from 2001–2011 (18).
Pulmonary coccidioidomycosis infections range from asymptomatic cases to fulminant pneumonia with severe respiratory compromise, or disseminated disease. The typical presenting symptoms include fever, cough, fatigue, and pleuritic chest pain. Within the endemic region 17–29% of community acquired pneumonia cases are caused by the Coccidioides species (19).
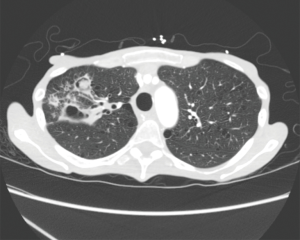
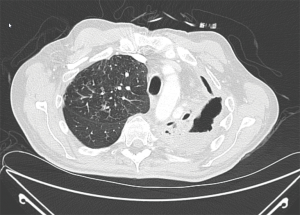
Chest X-ray and chest CT scan during acute infection may demonstrate lobar consolidation, parapneumonic effusions, or clusters of pulmonary nodules (Figure 3A). When the acute infection resolves, the pulmonary infiltrates or nodules may transform to thick or thin walled intra-parenchymal cavities (18). Some cavitary lesions resolve with antifungal therapy but others persist and progress over time despite oral fluconazole therapy (Figure 3B). Approximately 5–10% of coccidioidal infections develop residual or chronic pulmonary sequelae (20). Patients with chronic fibrocavitary disease are often symptomatic with fatigue, malaise, fever, night sweats, or weight loss. Serum tests for IgM and IgG antibodies to Coccidioidal antigens are often elevated in symptomatic patients but can be negative in cases of chronic pulmonary infection.

Coccidioidomycosis infections which are self-limited usually resolve with or without antifungal therapy. Cases presenting with severe symptoms or cases in high risk populations are treated with fluconazole therapy. Voriconazole and posaconazole are used in cases of fluconazole failure or intolerance (11,18,19).
Jaroszewski et al. and Ashfaq et al. published separate series of the coccidioidomycosis surgical experience at Mayo Clinic Hospital in Arizona. Surgery was performed for nodular disease, cavitary disease, pulmonary infiltrates, pneumothorax, hydropneumothorax, parapneumonic effusion, and empyema (20,21). Many of these patients had significant symptoms at the time of surgery but others required an operation to rule out malignancy. Nodular and cavitary disease are treated with pulmonary resection procedures such as a wedge resection, segmentectomy, or lobectomy. Complications of coccidioidomycosis such as parapneumonic effusion or empyema may require evacuation and decortication procedures (Figure 4). Pneumothorax and hydropneumothorax can be treated with a thoracostomy tube. However, in the event of a ruptured cavity, pulmonary resection and decortication may be required. Complex central cavities or chronic infection resulting in destroyed pulmonary parenchyma may require a pneumonectomy (Figure 5).
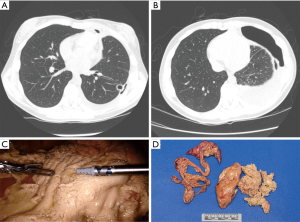
Open thoracotomy was the preferred surgical approach in the early cohort (1998–2009). Improved utilization of video assisted thoracoscopic surgery (VATS) reduced the thoracotomy rates to 5.2% in the contemporary cohort (2009–2012). Clinical outcomes and mortality rates for VATS procedures were equivalent or better when compared to open thoracotomy cases. In light of these results, VATS or alternative minimally invasive techniques are the preferred approach for appropriate patients at our institution (21). The most frequent post-operative complications in both surgical series was prolonged air leak and bronchopleural fistula (20,21).
Histoplasmosis
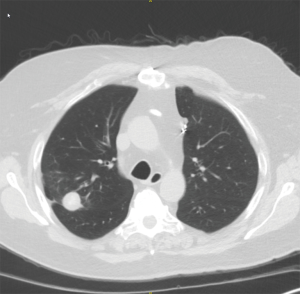
Histoplasmosis capsulatum is a dimorphic fungus which exists as a mold in the environment and as yeast in human tissue (22). The organism is highly endemic in the Mississippi and Ohio River Valleys, Central America, and South America. Patients with respiratory infection typically present with non-specific respiratory symptoms such as fever, chills, malaise, dyspnea, and cough. Chest radiograph findings vary significantly and can include either pulmonary or mediastinal findings. The most frequent finding is pulmonary or mediastinal lymph node calcifications which typically do not require intervention (23). However, enlarging pulmonary nodules, broncholithiasis, or fibrosing mediastinitis from histoplasmosis infection should prompt a thoracic surgery consultation. Pulmonary nodules are common in patients who live in endemic areas. Surgery for pulmonary nodules is primarily performed to rule out a pulmonary malignancy (19,22). The decision to biopsy or surgically resect a nodule depends on the size, growth, patient age, smoking status, and other patient risk factors including prior malignancy. Pulmonary resections for small peripheral nodules can be performed with minimal loss to overall pulmonary function. Minimally invasive techniques should be employed for such cases (Figure 6).
Broncholithiasis occurs when a calcified mediastinal lymph node erodes into an airway, causing intrinsic or extrinsic compression. The most common presenting symptoms include recurrent pulmonary infections or hemoptysis due to erosion into a bronchial artery or the pulmonary vasculature (Figure 7) (23). The indications for surgical treatment of broncholithiasis include intractable cough, persistent or massive hemoptysis, suppurative lung disease, bronchiectasis or bronchial stenosis, and fistulation. The location of the broncholith will guide the surgical approach. Central broncholiths can be removed with flexible or rigid bronchoscopy, but often the patient is prepped for surgical intervention in case a pulmonary artery injury occurs at removal. Other airway lesions can be treated with anatomic resection including segmentectomy, lobectomy, sleeve lobectomy/bronchoplastic procedures, or pneumonectomy (23,24).
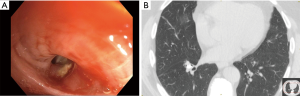
In a retrospective review of the broncholithiasis experience from Mayo Clinic, a total of 47 patients underwent surgical intervention over a fourteen year timespan. The intraoperative complication rate was 12.8% with pulmonary artery injury as most frequent event. Post-operative complications occurred in 34% of patients. These included hemothorax, empyema, respiratory insufficiency, prolonged air leak, segmental necrosis, and pulmonary artery thrombosis. There were no intraoperative or post-operative deaths in this patient cohort (24).
Fibrosing mediastinitis is a rare complication of histoplasmosis characterized by an invasive fibrotic process within the mediastinum resulting in fusing of tissue planes and ultimate obstruction of airways and vasculature (22). The disease is progressive and may remain asymptomatic for long periods of time with subsequent acute onset of severe symptoms. Patients can present with dyspnea or hemoptysis secondary to pulmonary artery occlusion. SVC occlusion is possible and may result in symptoms of SVC syndrome if there is not adequate venous collateralization. Surgical management includes placement of intravascular stents, airway stenting, superior vena cava reconstruction, and pneumonectomy for massive hemoptysis (23,25,26). Pneumonectomy in this patient population is high risk with mortality rates greater than 20% and should be avoided if possible (22,27).
Blastomycosis
Blastomyces dermatitidis is a dimorphic fungus which lives as a mold in soil and as a yeast in humans. In North America the primary areas for infection are the Great Lakes region and Ohio, Mississippi, and Saint Lawrence River Valleys. Symptoms of acute pulmonary blastomycosis include cough, fever, night sweats, weight loss, chest pain, dyspnea, myalgias, and hemoptysis. The clinical presentation varies widely from asymptomatic patients to those with fulminant pneumonia or acute respiratory distress syndrome (ARDS) (28,29).
Most blastomycosis infections are treated with medical management alone. Drug combinations of intravenous amphotericin B and oral itraconazole are used for various durations depending on infection severity and patient comorbid conditions (29). Surgical management is reserved to treat infectious complications or rule out malignancy. Acute pulmonary infections can be complicated by parapneumonic effusions or empyema which should be treated similar to other pleural infections with drainage and debridement of the pleural space (28). A case report by Wiesman et al. described successful management of a blastomycosis empyema via a minimally invasive surgical approach (30). Pulmonary nodules and mediastinal lymphadenopathy are typically the sequelae of prior infection and may be detected months to years after the primary pulmonary blastomycosis. These nodules present diagnostic challenges in high risk patient populations with no history of severe infection. Age, smoking history, prior infectious history, and oncologic history are important considerations when pondering diagnostic biopsy or resection.
Cryptococcosis
The primary infectious organisms causing cryptococcosis are Cryptococcus neoformans and Cryptococcus gattii. C. neoformans often presents in patients with human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) while C. gattii infects immunocompetent hosts primarily in the Pacific Northwest and portions of Canada (31). Clinical presentation is typically asymptomatic or has symptoms indistinguishable from other causes of pneumonia such as cough, fever, dyspnea, and chest pain. CNS involvement is common in cryptococcal disease and should be investigated. Up to 10% of patients with a resected pulmonary lesion develop cryptococcal meningitis after resection (32). Diagnosis can be challenging and surgery may be required to establish a diagnosis. Open lung biopsy of suspicious areas of infiltrates can be performed via minimally invasive or open techniques.
Most infections in immunocompetent patients are treated with fluconazole, with addition of amphotericin B in immunosuppressed patients, CNS Cryptococcus, or those with diffuse pulmonary disease (31). Surgical intervention is rare and usually reserved for complications of disease or to rule out malignancy in the case of residual enlarging pulmonary nodules.
Mucormycosis
Mucormycosis is a rare life-threatening fungal infection in immunocompromised patients caused by Rhizopus or Mucor species. The historical in-hospital mortality rate for isolated pulmonary mucormycosis is 65% and increases to 96% for patients with disseminated disease (33). At risk populations include patients with solid organ and bone marrow transplants, hematologic malignancies, diabetes, and steroid dependency. This angioinvasive organism causes tissue necrosis due to small vessel thrombosis resulting in cavitation and possible hemoptysis (34-36). Mucormycosis begins in the pulmonary parenchyma and bronchi but can spread rapidly to the pleura, diaphragm, chest wall, contralateral lung, or to extra-thoracic sites (Figure 8). The resultant necrotic lung lacks adequate blood supply limiting IV antifungal treatment efficacy and highlighting the need for surgical debridement.
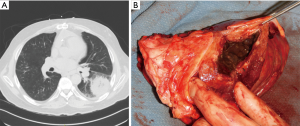
Initial presenting symptoms are similar to other pulmonary fungal infections including fever, dyspnea, hypoxia, chest pain, and hemoptysis (34-36). High clinical suspicion is essential as this infection progresses rapidly with resultant increased mortality rates. Contrasted CT scan of the chest is the diagnostic imaging modality of choice but findings may not differentiate Mucormycosis from other pulmonary fungal infections. Sputum cultures are often negative therefore bronchoscopy with bronchoalveolar lavage is preferred (34). CT guided transthoracic biopsy can also confirm a diagnosis. Prompt treatment with supportive therapy, reduction in immunosuppressive agents, antifungal therapy, and surgical debridement are essential for recovery.
Intravenous amphotericin B, isavuconazole, and posaconazole show activity against Mucorales strains in vitro (34,35). However, in vivo responses may be limited as the blood supply to the infected area is obliterated by the extensive thrombosis and tissue necrosis. Surgical resection is recommended in patients who are deemed to be operative candidates with disease limited to one lung. The surgical procedure of choice will depend on the extent of the disease. Wedge resections are rarely adequate with disease usually requiring a lobectomy or in some cases a pneumonectomy. If the disease extends outside the lung into adjacent pleura or chest wall, these areas also require debridement or resection.
Patients treated with a combined medical-surgical approach have better outcomes than those receiving medical therapy alone. Tedder et al. examined thirty patients at a single institution with pulmonary mucormycosis. Mortality rates declined from 68% with medical therapy alone to 11% in the combined medical-surgical group (33). Lee et al. reported a 55% mortality rate in PM patients receiving antifungal therapy and 27% mortality in surgically resected patients. Hematologic malignancy and neutropenia were poor prognostic indicators (37).
Summary
Surgical therapy is an important treatment modality in non-mycobacterial pulmonary fungal disease. The clinical spectrum includes asymptomatic patients with post infectious pulmonary nodules and those with diseased lungs, severe illness, and rapidly progressing infection. The operative plan has equal variability and surgeons treating these patients need to perform a range of complex thoracic procedures and identify strategies to reduce post procedure complications. Although operative risk is important to consider, the results of many studies show improved survival in patients receiving both medical and surgical therapy, and thus should be considered in appropriate candidates. Risk assessment and counseling for these patients and their families is essential.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Jaroszewski DE, Webb BJ, Leslie KO. Diagnosis and Management of Lung Infections. Thorac Surg Clin 2012;22:301-24. [Crossref] [PubMed]
- Mitchell J. Infectious Lung Diseases. In: Selke F, del Nido P, Swanson S. editors. Sabiston and Spencer Surgery of the Chest. Philadelphia: Elsevier, 2016:205-26.
- Passera E, Rizzi A, Robustellini M, et al. Pulmonary Aspergilloma: Clinical Aspects and Surgical Treatment Outcomes. Thorac Surg Clin 2012;22:345-61. [Crossref] [PubMed]
- Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73. [Crossref] [PubMed]
- Moodley L, Pillay J, Dheda K. Aspergilloma and the surgeon. J Thorac Dis 2014;6:202-9. [PubMed]
- Babatasi G, Massetti M, Chapelier A, et al. Surgical treatment of pulmonary aspergilloma: current outcome. J Thorac Cardiovasc Surg 2000;119:906-12. [Crossref] [PubMed]
- Regnard JF, Icard P, Nicolosi M, et al. Aspergilloma: A series of 89 surgical cases. Ann Thorac Surg 2000;69:898-903. [Crossref] [PubMed]
- Jewkes J, Kay P, Paneth M, et al. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax 1983;38:572-8. [Crossref] [PubMed]
- He G, et al. Intervention treatment on massive hemoptysis of pulmonary aspergilloma. Exp Ther Med 2017;13:2259-62. [Crossref] [PubMed]
- Musher B, Fredricks D, Leisenring W, et al. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J Clin Microbiol 2004;42:5517-22. [Crossref] [PubMed]
- Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 2011;183:96-128. [Crossref] [PubMed]
- Massard G, Roeslin N, Wihlm JM, et al. Pleuropulmonary aspergilloma: clinical spectrum and results of surgical treatment. Ann Thorac Surg 1992;54:1159-64. [Crossref] [PubMed]
- Chen QK, Chen C, Chen XF, et al. Video-Assisted Thoracic Surgery for Pulmonary Aspergilloma: A Safe and Effective Procedure. Ann Thorac Surg 2014;97:218-223. [Crossref] [PubMed]
- Cesar JM, Resende JS, Amaral NF, et al. Cavernostomy x resection for pulmonary aspergilloma: a 32-year history. J Cardiothorac Surg 2011;6:129. [Crossref] [PubMed]
- García-Yuste M, Ramos G, Duque JL, et al. Open-window thoracostomy and thoracomyoplasty to manage chronic pleural empyema. Ann Thorac Surg 1998;65:818-22. [Crossref] [PubMed]
- Akbari JG, Varma PK, Neema PK, et al. Clinical profile and surgical outcome for pulmonary aspergilloma:a single center experience. Ann Thorac Surg 2005;80:1067-72. [Crossref] [PubMed]
- Park CK, Jheon S. Results of surgical treatment for pulmonary aspergilloma. Eur J Cardiothorac Surg 2002;21:918-23. [Crossref] [PubMed]
- Twarog M, Thompson GR III. Cocciodiomycosis: Recent updates. Semin Respir Crit Care Med 2015;36:746-55. [Crossref] [PubMed]
- Thompson GR 3rd. Pulmonary Coccidiomycosis. Semin Respir Crit Care Med 2011;32:755-63. [Crossref]
- Jaroszewski DE, Halabi WJ, Blair JE, et al. Surgery for Pulmonary Coccidiomycosis: A 10-Year Experience. Ann Thorac Surg 2009;88:1765-72. [Crossref] [PubMed]
- Ashfaq A, Vikran HR, Blair JE, et al. Video-assisted Thoracoscopic Surgery for Patients with Pulmonary Coccidiomycosis. J Thorac Cardiovasc Surg 2014;148:1217-23. [Crossref] [PubMed]
- Hage CA, Azar MM, Barh N, et al. Histoplasmosis: Up-to-date Evidence-Based Approach to Diagnosis and Management. Semin Respir Crit Care Med 2015;36:729-45. [Crossref] [PubMed]
- Hammoud ZT, Rose AS, Hage CA, et al. Surgical Management of Pulmonary and Mediastinal Sequelae of Histoplasmosis: A Challenging Spectrum. Ann Thorac Surg 2009;88:399-403. [Crossref] [PubMed]
- Potaris K, Miller DL, Trastek VF, et al. Role of Surgical Resection in Broncholithiasis. Ann Thorac Surg 2000;70:248-51; discussion 251-2. [Crossref] [PubMed]
- Doyle TP, Loyd JE, Robbins IM. Percutaneous pulmonary artery and vein stenting: a novel treatment for mediastinal fibrosis. Am J Respir Crit Care Med 2001;164:657-60. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Long-term results of fiberoptic bronchoscopic balloon dilation in the management of benign tracheobronchial stenosis. Chest 1998;114:796-800. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC. Clinical manifestation of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg 1992;54:1053-7; discussion 1057-8. [Crossref] [PubMed]
- LoCicero J 3rd, Shaw JP, Lazzaro RS. Surgery for Other Pulmonary Fungal Infections, Actinomyces, and Nocardia. Thorac Surg Clin 2012;22:363-74. [Crossref] [PubMed]
- Smith JA, Gauthier G. New Developments in Bastomycosis. Semin Respir Crit Care Med 2015;36:715-28. [Crossref] [PubMed]
- Wiesman IM, Podbielski FJ, Hernan MJ, et al. Thoracic Blastomycosis and Empyema. JSLS 1999;3:75-8. [PubMed]
- Chang CC, Sorrell TC, Chen SC. Pulmonary Cryptococcosis. Semin Respir Crit Care Med 2015;36:681-91. [Crossref] [PubMed]
- Metlay JP, Fine MJ. Testing strategies in the initial management of patients with community-acquired pneumonia. Ann Intern Med 2003;138:109-18. [Crossref] [PubMed]
- Tedder M, Spratt JA, Anstadt MP, et al. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg 1994;57:1044-50. [Crossref] [PubMed]
- Danion F, Aguilar C, Catherinot E, et al. Mucormycosis: New Developments into a Persistently Devastating Infection. Semin Respir Crit Care Med 2015;36:692-705. [Crossref] [PubMed]
- Hamilos G, Saminis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med 2011;32:693-702. [Crossref] [PubMed]
- Merritt RE, Shrager JB. Indications for surgery in patient with localized pulmonary infection. Thorac Surg Clin 2012;22:325-32. [Crossref] [PubMed]
- Lee FY, Mossad FB, Adal KA, et al. Pulmonary mucormycosis the last 30 years. Arch Intern Med 1999;159:1301-9. [Crossref] [PubMed]


