Practice of diagnosis and management of acute respiratory distress syndrome in mainland China: a cross-sectional study
Introduction
The American European Consensus Conference (AECC) definition (1) was the first widely accepted definition of acute respiratory distress syndrome (ARDS). However, it had a number of limitations, and as a result, a new consensus definition of ARDS—the Berlin definition—was developed in 2012 (2). This new definition stipulated that the oxygenation criterion had to be obtained using a minimum positive end-expiratory pressure (PEEP) of 5 cmH2O. It was suggested that this could lead to changes in recognition and outcomes of patients with ARDS (3). Previous data demonstrated that 5–15% of ARDS patients diagnosed by the AECC definition would not fulfill the Berlin definition as PEEP >5 cmH2O could change the P/F ratio, one of the cornerstones of the ARDS diagnosis (2). Implementation of effective therapies may be limited by the lack of recognition of ARDS by clinicians (4,5). Hence, understanding the implications of the two definitions for ARDS and the effect on management may help improve our appreciation of how clinicians care for patients with ARDS.
Lung-protective ventilation strategies, commonly used in patients with ARDS (6), focus on limiting tidal volume (Vt) and plateau pressure (Pplat) while providing adequate PEEP to limit ventilator-induced lung injury (VILI) (7-11). A number of adjunctive interventions, such as recruitment manoeuvers (RM) (12-14), prone positioning, (15) neuromuscular blockade, (16) and extracorporeal membrane oxygenation (ECMO) (17) for ARDS patients have been proposed. Implementation of these interventions may be affected by local ICU culture and intensive care resources. The LUNG SAFE study demonstrated geographic variations in ARDS management (18) would lead to different outcomes in similar patient populations.
As a middle-income county, critical care medicine developed rapidly in Mainland China in the past three decades. However, there has been little information about the incidence of ARDS in mainland China, and the management of ARDS has still been unclear. Understanding the practice of diagnosis and management of ARDS could lead to effective interventions to improve care. Therefore we conducted a one month, national, prospective observational cohort study to determine the current diagnosis base on AECC and Belin definitions and outcome of ARDS, and understand how clinicians use mechanical ventilation and adjunctive interventions in routine clinical practice in mainland China.
Methods
Study design
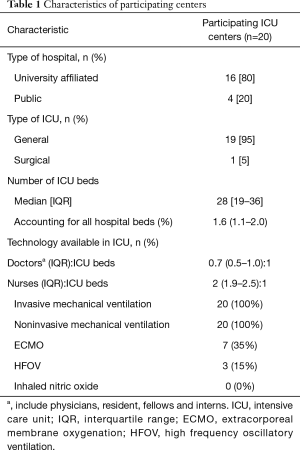
The study was a one-month (August 31st 2012, to, September 30th, 2012) prospective, observational study to describe the diagnosis and management of ARDS in 20 participating Chinese ICUs. All participating centers were closed ICUs in tertiary teaching hospitals in metropolitan cities, managed by full-time ICU directors. The doctor/bed ratio and nurse/bed ratio was 0.6:1 and 2:1 in the participating ICUs (Table 1). The protocol was approved by Institutional Ethics Committee of the Zhongda hospital (the core center, Approval No. 2012ZD11KY09.0). Informed consent was waived due to the observational nature of the study. The trial was registered at clinicaltrials.gov (NCT01666834).
Full table
Study population
All patients admitted to the participating ICUs during the study period were screened by the researchers at 8 a.m. during the morning round for the diagnosis of ARDS based on the diagnostic criteria of the Berlin Definition (2) or AECC definition (1). Exclusion criteria were age less than 18 years and an ARDS diagnosis before the enrollment period (August 31st 2012).
Data collection
For every enrolled patient, clinical data were assessed to detect whether they fulfilled either the AECC criterion or the Berlin criterion or both. Demographic data, severity of illness and causes of ARDS or acute lung injury (ALI) were recorded. Day 1 was defined as the first day which the patients fulfilled the ARDS or ALI criteria, irrespective of ICU admission date. Acute Physiology and Chronic Health Evaluation (APACHE) II score (19), ventilator settings, breathing pattern, blood gases were recorded on day 1 and day 3. Adjunctive interventions including recruitment manoeuvers, prone positioning, neuromuscular blockade, glucocorticoids administration, NO inhalation, high frequency oscillatory ventilation (HFOV) and ECMO were recorded during the study period. All data were recorded at 9 am each day, and the mean value of respiratory parameters in one minute was recorded. During controlled ventilation (no spontaneous effort, set and measured respiratory rate equal), driving pressure (∆P) was defined as Pplat minus PEEP. Given the difficulty in comparing non-invasive ventilation settings to invasive modes, we excluded patients ventilated on non-invasive ventilation from the analyses of ventilator management. The predicted body weight of male and female patients was calculated according to the ARDSnet calculation (7).
Enrolled patients were categorized on the day of ARDS diagnosis based on their PaO2/FIO2 ratio into mild, moderate, or severe, based on the Berlin definition, and were categorized into ALI without ARDS and with ARDS based on the AECC definition. Patients who died before weaning was attempted were considered to have a ventilator-free-day value of 0.
Outcome measures
All enrolled patients were followed until one of the following events occurred, whichever happened first: discharge from the hospital, death on the current hospital admission.
Statistical analysis
Statistical analysis was performed with SPSS 17.0 (IBM, USA). Normally distributed data are presented as mean ± SD and non-normally distributed data are presented as median (interquartile range). Comparisons of proportions were made using Pearson Chi-Square or exact Fisher tests. Continuous variables were compared with the use of the one way ANOVA or Mann-Whitney test, as appropriate. Student-Newman-Keuls post hoc comparison test was used to compare variables between groups. Linear regression and Pearson product-moment correlation was used to detect whether Vt and PEEP changed with respiratory compliance. A Kaplan-Meier estimate of the cumulative probability of survival to day 28 was performed. The ROC curve was used to evaluate the predictive value of AECC and Berlin criteria for ICU mortality. Significant difference was defined as P<0.05.
Results
Patients enrolled and characteristics
There were a median of 28 [19–36] beds in each ICU, accounting for 1.6% (1.1–2.0%) of all hospital beds. ECMO and HFOV were available in 7 (35%) and 3 (15%) ICUs, respectively (Table 1).
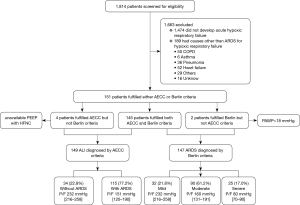
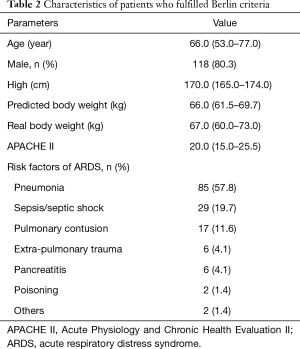
Of the 1,814 patients admitted during the enrollment period, 149 (8.2%) patients were diagnosed with ALI by AECC criteria, and 147 (8.1%) with ARDS by Berlin criteria (Figure 1). Since the Berlin definition is most widely used currently, we analyzed the mechanical ventilation data based on the Berlin definition only. Table 2 outlines key characteristics of the 147 ARDS patients who fulfilled Berlin criteria. Pneumonia (57.8%), sepsis/septic shock (19.7%) and trauma (11.6%) were the main risk factors for ARDS.
Full table
Mechanical ventilation in ARDS
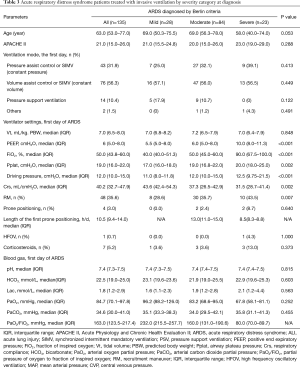
Twelve patients (8.1%) with ARDS who received noninvasive ventilation were excluded from analyses. As shown in Table 3, ventilation mode and Vt were comparable in the three groups (P>0.05). Cumulative frequency distribution of Vt was similar in patients in each severity category (Figure 2A), with 75.6% of patients with ARDS receiving a Vt of 8 mL/kg predicted body weight or less. Median Pplat was 17.0, 19.0 and 20.0 cmH2O in mild, moderate and severe ARDS patients (P=0.002). Ninety four (75.2%) patients received protective mechanical ventilation as defined by a Vt ≤8 mL/kg PBW and a Pplat ≤30 cmH2O.
Full table
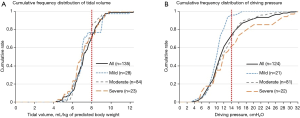
The cumulative distributions for ∆P were similar at lower levels of ∆P, but diverged at higher levels of ∆P. In mild ARDS, about 80% of the patients had a ∆P ≤12 cmH2O, in moderate ARDS, 80% had a ∆P ≤15 cmH2O, and in severe ARDS 80% had a ∆P ≤18 cmH2O (Figure 2B). The increase in ∆P with severity category was due to the decrease of Crs rather than an increase in Vt (Table 3). There was a positive correlation between Vt and Crs (Figure 3A) (R2=0.195, P<0.001); however, no correlation was found between PEEP and Crs (Figure 3B) (R2=0.001, P=0.706). Hypercapnia, as defined by a PaCO2 above 45 mmHg, was observed in 16 (11.9%) patients.

PEEP levels were relatively low (Table 3) with a median PEEP level of 10.0 (8.0–11.3) cmH2O in severe ARDS patients; these patients had a median FiO2 of 90% (Table 3). Less than 10% of the patients received PEEP levels that were 12 cmH2O or greater. Except for the PaO2/FiO2 ratio blood gas values did not vary with ARDS severity (Table 3).
Adjunctive measures
The use of adjunctive treatments in patients with ARDS was relatively low. Recruitment maneuvers were the most frequently used adjunct measure performed in 34.8% of the patients; these maneuvers were used more often in patients with severe ARDS than mild ARDS (Table 3). Overall, only 3.0% patients received prone position, and in the most severe group this number was 8.7%. Steroids, HFOV, ECMO, neuromuscular blockers or nitric oxide inhalation were rarely or no used during the study period (Table 3).
ARDS outcomes
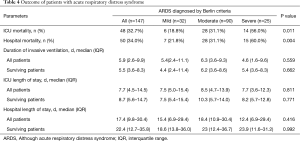
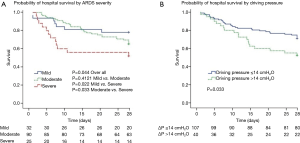
Overall, unadjusted ICU and hospital mortality from the 147 ARDS patients who fulfilled Berlin criteria were 32.7% and 34.0%, respectively (Table 4). There was a decreased likelihood of survival at day 28 with increasing ARDS severity (Figure 4A). Patients with a ∆P >14 cmH2O on day 1 had a worse outcome (Figure 4B). The Berlin and AECC definition had similar predictive validity for mortality with an area under the receiver operating curve of 0.623 (95% CI, 0.523–0.724) vs. 0.574 (95% CI, 0.473–0.674) (P=0.462).
Full table
Discussion
In the present study, we found a higher hospital mortality of severe ARDS in mainland China than other countries (20). Meanwhile the management of ARDS was different in a number of respects. (I) Lung protective strategy using low Vts and limiting Pplat was generally accepted, and Vt was positive correlated with Crs. (II) PEEP levels were relatively low especially in severe ARDS patients, and no evidence of higher PEEP was found in patients with a low Crs. (III) The use of adjunctive treatment in patients with ARDS was relatively low, especially the use of ECMO and HFOV. (IV) Similar patient-diagnosed and predictive validity for mortality were found between Berlin and AECC definition.
We found a very small difference for diagnosis and no difference for outcome prediction between the Berlin and AECC definitions. Unavailable PEEP and PAWP >18 mmHg were the main exclusion factors for Berlin and AECC, respectively, however, distinction of clinical diagnosis and outcome was not obvious. The prevalence of ARDS in Chinese ICUs was 8.1%, which may be under-recognized by physicians. It has been demonstrated that ARDS continues to be under-recognized by clinicians in the era of the Berlin Definition, with 40% of all cases not being diagnosed (18). This is similar to previous findings using the AECC definition (5,21-23). However, the present study did not set out to assess the degree of under-recognition.
ARDS is commonly managed by invasive mechanical ventilation and the lung protective strategy of low Vt and limited Pplat was generally accepted, with 75% of patients in the present study receiving a Vt <8 mL/kg PBW and a Pplat ≤30 cmH2O. However, similar to the LUNG SAFE study, only less than 15% of the patients receiving a Vt <6 mL/kg PBW in the present study (18). As reported in prior studies (22), Vt was constant across the spectrum of ARDS severity, accordingly the ∆P increased with the ARDS severity. However, the difference of median ∆P between mild and severe ARDS was only 1.5 cmH2O, which did not make sense clinically. It has been demonstrated that airway ∆P can detect lung overstress with an acceptable accuracy (24). The optimal cutoff values for ∆P were 15 cmH2O considering a stress equal or above 24 cmH2O (24). There are 21% patients with a ∆P more than 14 cmH2O in the present study, however, only 1.6% patients with a Pplat more than 30 cmH2O. This might be induced by the low PEEP level. It may be reasonable to using ∆P as an additional safety threshold for Vt setting.
Although PEEP increased significantly with higher ARDS severity, it was relatively low, with 90% of patients receiving a PEEP <12 cmH2O. We found no evidence that a higher PEEP was used in patients with lower respiratory system compliance. At present, the aim of PEEP is not only to improve oxygenation but also to minimize VILI by limiting tidal alveolar opening and collapse (25). Hypoxemia appears to be treated predominantly by an increased FiO2. In severe ARDS the median PEEP level was 10.0 cmH2O and the median FiO2 was 90%. This may be partly explained by the lack of application of the PEEP/FiO2 table by the ARDS Network investigators with higher PEEP levels (26,27). At present, the only such protocol is the PEEP/FiO2 table proposed by the ARDS Network (ARDSNet) (7), which likely tolerates minor atelectasis by applying a minimal PEEP and FiO2 to match an acceptable arterial oxygenation target (between 55 and 80 mmHg). Although the ARDSnet PEEP/FiO2 table was widely known by the intensivist, however the compliance of this table was still poor in mainland China. In the present study, 23 severe ARDS patients came from 16 ICUs. However, compared with the PEEP/FiO2 table, low PEEP was used in 12 out of the 16 ICUs, and only 4 ICUs used acceptable PEEP level in severe ARDS patients.
ARDS appears to be undertreated in China as judged by the use of adjunctive measures. The effectiveness of recruitment remains controversial in patients with ARDS (26-30). However, the relatively low use of less expensive interventions such as prone positioning and neuromuscular blockade is unclear because the evidence in support of these approaches is better than the evidence for recruiting maneuvers. Probably due to lack of available equipment, expensive and invasive adjunctive measures such as ECMO and HFOV were uncommonly used in the participating ICUs.
The hospital mortality in severe ARDS in mainland China was extremely high (60%), which might be a result of low PEEP level, high ∆P, and low use of adjunctive treatment such as prone position, neuromuscular blockers and ECMO. Inadequate PEEP caused deterioration of lung compliance, which might be the main reason of the higher ∆P in the severe ARDS group. In agreement with other study (31), we confirmed that ARDS patients with high ∆P (>14 cmH2O) had shorter survival times than patients with lower ∆P (≤14 cmH2O).
The present study has several limitations. First, the time for the observation period was arbitrarily chosen to summer, which may underestimate the ICU incidence of ARDS. Second, the diagnosis of ARDS was made by the physician in charge; however, it has been shown that only about 60% of all patients with ARDS are clinician-recognized (18). Unfortunately, the raw data that made up the various components of the Berlin ARDS Definition were unavailable in the present study to address this issue. Third, two patients who fulfilled the Berlin criteria were excluded by AECC criteria due to PAWP >18 mmHg. However PAWP were measured only in 19 enrolled patients, this could constitute a concerning bias. Fourth, we enrolled 20 ICUs from 9 provinces in China only, and our sample may be prone to selection biases that may limit generalizability. Fifth, we only focus on the respiratory aspects and did not show the hemodynamic impairment of ARDS patient. Finally, though the LUNG SAFE study gave important information concerning the ARDS patients (18), the medical system in mainland China is far different form the west which will lead to the different management and outcome of ARDS patients. Our data will help to improve the management of ARDS in mainland China.
Conclusions
Despite the lung protective strategy using low Vts and limitation of Pplats was generally accepted in mainland China, ∆P is still a matter should be concerned. High PEEP and adjunctive treatments were used relatively infrequently in mainland China. The Berlin and AECC definitions were similar in terms of diagnoses and predictive validity for mortality. These data may serve as a current benchmark on the usual care and outcomes of patients with ARDS in mainland China. It may also indicate the potential for improvement in management.
Acknowledgements
Funding: This study was supported by The Ministry of Health of China Special Fund for Health-scientific Research in the Public Interest Program (grant number 201202011), Clinical Science and Technology Specific Projects of Jiangsu Province (grant number BL2013030) and Natural Science Foundation of China (grant numbers 81670074, 81471843, 81571874).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Ethics Committee of the Zhongda hospital (No. 2012ZD11KY09.0). Informed consent was waived due to the observational nature of the study.
References
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet 2016;388:2416-30. [Crossref] [PubMed]
- Needham DM, Yang T, Dinglas VD, et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med 2015;191:177-85. [Crossref] [PubMed]
- Fröhlich S, Murphy N, Doolan A, et al. Acute respiratory distress syndrome: underrecognition by clinicians. J Crit Care 2013;28:663-8. [Crossref] [PubMed]
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012;38:1573-82. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Amato MBP, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Villar J, Kacmarek RM, Perez-Mendez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311-8. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010;303:865-73. [Crossref] [PubMed]
- Ong ME, Lee Ng CH, Goh K, et al. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Crit Care 2012;16:R108. [Crossref] [PubMed]
- Lachmann B. Open up the lung and keep the lung open. Intensive Care Med 1992;18:319-21. [Crossref] [PubMed]
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006;354:1775-86. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. [Crossref] [PubMed]
- Villar J, Blanco J, Añón JM, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 2011;37:1932-41. [Crossref] [PubMed]
- Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: under recognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med 2005;33:2228-34. [Crossref] [PubMed]
- Kalhan R, Mikkelsen M, Dedhiya P, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med 2006;34:300-6. [Crossref] [PubMed]
- Herasevich V, Yilmaz M, Khan H, et al. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med 2009;35:1018-23. [Crossref] [PubMed]
- Chiumello D, Carlesso E, Brioni M, et al. Airway driving pressure and lung stress in ARDS patients. Critical Care 2016;20:276. [Crossref] [PubMed]
- Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 2006;32:24-33. [Crossref] [PubMed]
- Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:637-45. [Crossref] [PubMed]
- Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795-805. [Crossref] [PubMed]
- Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646-55. [Crossref] [PubMed]
- Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-36. [Crossref] [PubMed]
- Suzumura EA, Figueiró M, Normilio-Silva K, et al. Effects of alveolar recruitment maneuvers on clinical outcomes in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intensive Care Med 2014;40:1227-40. [Crossref] [PubMed]
- Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]


