Clinical differences between pulmonary and extrapulmonary acute respiratory distress syndrome: a retrospective cohort study of prospectively collected data in Japan
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening condition with a poor prognosis. ARDS reportedly exhibits various clinical phenotypes with different risk and prognostic factors (1,2). Recently, a retrospective study reported that, although patients with pulmonary ARDS and those with extrapulmonary ARDS exhibited similar mortality, they showed significantly different mortality predictors (3). However, few studies have evaluated these different phenotypes since publication of the Berlin definition of ARDS in 2012 (4). The objectives of the present study were to determine the characteristics of pulmonary and extrapulmonary ARDS and explore differences in prognostic factors between the two phenotypes.
Methods
Patients
This single-center study involved the retrospective analysis of data collected during an ongoing prospective cohort study assessing ARDS with high-resolution computed tomography (HRCT), some of which have been previously published (5-8). In total, 200 Japanese patients with ARDS were admitted to our hospital between October 2004 and September 2017. Our hospital is an urban acute medicine teaching hospital with a capacity of 400 beds. ARDS was diagnosed according to the Berlin definition (4). We also reviewed cases diagnosed before publication of the Berlin definition and assessed whether they met the diagnostic criteria. The patients were classified into pulmonary and extrapulmonary ARDS groups according to the underlying risk factors. Patients with pneumonia and aspiration as risk factors and those with pulmonary sepsis were assigned to the pulmonary ARDS group, whereas those with non-pulmonary sepsis and other risk factors (e.g., trauma, pancreatitis, massive blood transfusion) were assigned to the extrapulmonary ARDS group.
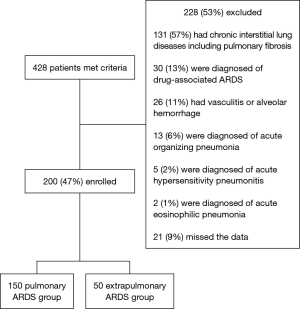
Written informed consent was obtained from all patients or their families. The study was approved by our institutional review board (approval number, 238), and the study was conducted in accordance with the ethical standards of the Declaration of Helsinki. Figure 1 shows the study flowchart. Patients with drug-associated ARDS; chronic interstitial lung disease, including idiopathic pulmonary fibrosis, vasculitis, and alveolar hemorrhage; acute organizing pneumonia; hypersensitivity pneumonitis; and/or acute eosinophilic pneumonia were excluded.
Treatment
Mechanical ventilation and weaning from ventilation were implemented according to evidence-based guidelines, with reference to the lower tidal volume (VT) strategy and predicted body weight (PBW) (6< VT <10 mL/kg PBW) in the ARDS Clinical Trial (9), and guidelines for weaning and discontinuing ventilatory support published by the American College of Chest Physicians (10). PBW was calculated as 49.9+0.91 [height (cm)—152.4] for men and 45.4+0.91 [height (cm)—152.4] for women (9). The plateau pressure was limited to <30 cmH2O, with a positive end-expiratory pressure (PEEP) of 8–12 cmH2O. PEEP, peak inspiratory pressure (PIP), and VT were recorded on the first hospitalization day. High-dose corticosteroid therapy was defined when a dose of >2 mg/kg/day was administered. Two patients with pulmonary ARDS received extracorporeal membrane oxygenation.
Comparison of prognostic factors and outcome measures
The pulmonary and extrapulmonary ARDS groups were compared for the patient age, sex, 60-day mortality, the duration of mechanical ventilation, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, sequential organ failure assessment (SOFA) scores, HRCT scores (extent of fibroproliferation), disseminated intravascular coagulation (DIC) scores, arterial oxygen tension (PaO2)/fractional inspired oxygen (FiO2), and blood test findings. As previously published (11), the HRCT score was assigned on a 6-point scale: (I) normal attenuation; (II) ground-glass attenuation; (III) consolidation; (IV) ground-glass attenuation with traction bronchiolectasis or bronchiectasis; (V) consolidation with traction bronchiolectasis or bronchiectasis; and (VI) honeycombing. The presence of each of these six abnormalities in the upper, middle, and lower segments of each lung was independently assessed. The extent of each abnormality was determined by visually estimating the percentage of the affected lung parenchyma in each segment. Each abnormality score was calculated by multiplying the percentage area by each score. Scores for the six segments were averaged to determine the total score for each abnormality. The overall HRCT score was derived by adding the six abnormality scores. This visual estimation method was further validated (5). Additionally, in the study of acute exacerbation of idiopathic pulmonary fibrosis, this score reportedly predicted mortality (12). The DIC score was calculated in accordance with the diagnostic criteria by the Japanese Association of Acute Medicine (13). The primary and secondary outcomes were 60-day mortality and the duration of mechanical ventilation, respectively.
Statistical analysis
Continuous variables are expressed as medians and interquartile ranges (IQRs). In univariate analysis for the two groups, categorical variables were compared using the χ2 test or Fisher’s exact test, while continuous variables were compared using the Mann-Whitney U test. For assessing 60-day mortality and the duration of mechanical ventilation, multivariate analysis was performed using a Cox proportional hazard model with Akaike’s Information Criterion. Subsequently, we simultaneously added pulmonary or extrapulmonary ARDS to the model. Variables with an r value of >0.4 in the factor analysis were excluded from multivariate analysis. Unadjusted and adjusted survival curves were plotted using the Kaplan-Meier method. Log-rank tests were used to compare differences in survival. The time to successful discontinuation of mechanical ventilation was also evaluated as previously described (14). We also estimated adjusted relationships between pulmonary ARDS and outcomes using a Cox proportional hazards regression model via inverse probability of treatment weighting (IPTW) with a propensity score. The propensity score model for pulmonary ARDS versus extrapulmonary ARDS was constructed using a logistic regression model including age, sex, white blood cell (WBC) counts, C-reactive protein levels, serum lactate dehydrogenase (LDH) levels, albumin levels, APACHE II scores, SOFA scores, HRCT scores, DIC scores, and PaO2/FiO2 as the main variables. For analysis of the predictors of 60-day mortality in both groups, we performed univariate analysis using a Cox proportional hazards regression model. Then, WBC counts, serum LDH levels, and PaO2/FiO2 were converted from continuous variables to categorical variables using cut-off values based on median values. We used the EZR software (Saitama Medical center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R VV.3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria), for all statistical analyses (15) and SPSS software (V.22.0) for the factor analysis. A P value of <0.05 was considered statistically significant.
Results
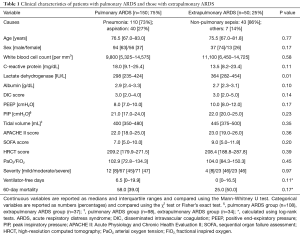
There were 150 and 50 patients in the pulmonary and extrapulmonary ARDS groups, respectively. Table 1 shows the etiologies and clinical characteristics of patients in both groups.
Full table
Prognostic implications
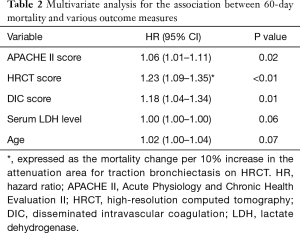
Death within 60 days was recorded for 58 (39%) and 25 (50%) patients in the pulmonary and extrapulmonary ARDS groups, respectively. Figure 2A shows Kaplan-Meier survival curves plotted at 60 days for both groups. There was no significant difference in 60-day mortality between groups (39% vs. 50%, P=0.17). The duration of ventilation was also comparable between groups (21.5 vs. 28 days, P=0.11; Figure 2B). Both APACHE II and DIC scores correlated with SOFA scores (r=0.53 and 0.45, respectively). Therefore, we excluded the SOFA score from multivariate analysis, which revealed that APACHE II [hazard ratio (HR), 1.06; 95% CI, 1.01–1.11; P=0.02], HRCT (HR, 1.23; 95% CI, 1.09–1.35; P<0.01), and DIC (HR, 1.18; 95% CI, 1.04–1.34; P=0.01) scores were independently associated with 60-day mortality (Table 2).

Full table
Further, pulmonary or extrapulmonary ARDS was not associated with mortality (adjusted HR, 1.00; 95% CI, 0.61–1.64; P=0.99; Figure 3A) and the duration of ventilation (adjusted HR, 1.21; 95% CI, 0.74–1.99; P=0.45; Figure 3B). IPTW estimators with propensity score adjustment also showed that pulmonary or extrapulmonary ARDS was not associated with mortality (HR, 0.75; 95% CI, 0.46–1.24; P=0.30) and the duration of mechanical ventilation (HR, 1.63; 95% CI, 0.79–3.37; P=0.22). The area under the curve for the calculated propensity score was 0.68 (95% CI, 0.60–0.77).

Comparison of prognostic factors
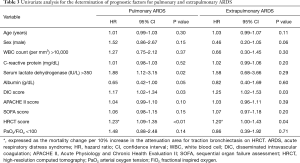
There was no significant difference in any parameter except the serum LDH level, which was higher in the extrapulmonary ARDS group (298 vs. 364 IU/mL, P=0.01). Univariate analysis for the predictors of 60-day mortality in both groups revealed that HRCT and DIC scores were significant predictors in both groups, whereas the serum LDH level was a predictor only in the pulmonary ARDS group (Table 3).
Full table
Discussion
This single-center cohort study investigated clinical differences between pulmonary and extrapulmonary ARDS. There were no significant differences between groups about corticosteroid therapy (P=0.84) and variables of mechanical ventilation, and there were no significant differences in 60-day mortality or duration of mechanical ventilation between groups. In the present study, serum LDH level was significantly higher in the extrapulmonary ARDS group than pulmonary ARDS group. Although HRCT score, which indicates fibroproliferative lesions, was not significantly different between the two groups, lung damage may have been slightly worse in the extrapulmonary ARDS group.
APACHE II, HRCT, and DIC scores were associated with 60-day mortality and a shorter duration of mechanical ventilation. HRCT and DIC scores were associated with 60-day mortality in both groups. Both APACHE II and DIC scores correlated with the SOFA score, which is one of the most common organ dysfunction scoring system.
Clinically, older ARDS patients show higher morbidity rates, require prolonged mechanical ventilation, and show a poorer prognosis than do younger patients (16). Age-related differences in mortality and outcomes have been attributed to a higher number of comorbidities and a higher frequency of non-pulmonary organ system failure in older patients. Although aging reportedly increases the susceptibility to lung injury and pulmonary fibroproliferation via a potential redox imbalance (17), further investigation of younger patients with ARDS should validate our study results.
Studies conducted before publication of the Berlin definition reported no differences in mortality between pulmonary and extrapulmonary acute lung injury/ARDS (18-20). A recent retrospective cohort study reported that, although the mortality rate was similar, the clinical predictors of hospital mortality differed between pulmonary and extrapulmonary ARDS (3); hospital mortality was associated with age, the lung injury score, and the number of failed organs in patients with pulmonary ARDS and only the number of failed organs in patients with extrapulmonary ARDS. In the present study, as expected, there was no significant difference in mortality between pulmonary and extrapulmonary ARDS groups. Moreover, our findings were consistent with those in Luo’s study (3), where 60-day mortality was associated with the HRCT score and serum LDH level, which suggest the degree of lung injury, in the pulmonary ARDS group, and the DIC score, which suggests multiorgan failure, in the pulmonary and extrapulmonary ARDS groups. The HRCT score was also associated with 60-day mortality in the extrapulmonary ARDS group in our study.
DIC involves the systematic activation of blood coagulation, which results in fibrin formation and composition. Minute thrombin generation causes organ ischemia in various organs and often leads to multiorgan failure. The Japanese Association for Acute Medicine proposed the DIC scoring system, which reportedly has eminent value as a predictor of multiple organ dysfunction syndrome and a poor prognosis in patients with severe sepsis (21). In the present study, the DIC score was useful to assess the prognosis of patients with pulmonary or extrapulmonary ARDS, consistent with findings in a previous study reporting that the number of failed organs was associated with hospital mortality in these patients (3). These findings suggest that the DIC score is a very important prognostic factor for ARDS.
HRCT findings correlate with the pathological stage of diffuse alveolar damage. Ichikado et al. reported that HRCT scores were associated with not only a poor prognosis and prolonged mechanical ventilation but also multiorgan failure and ventilator-associated complication (barotrauma and ventilator-associated pneumonia) (5). In the present study, the HRCT score was useful to assess prognosis with pulmonary or extrapulmonary ARDS. This score was associated with mortality in the pulmonary ARDS group, consistent with the finding in a previous study reporting that the lung injury score was associated with hospital mortality in the pulmonary ARDS group (3). That study also reported that the HRCT score, but not the lung injury score, was associated with hospital mortality in the extrapulmonary ARDS group. A possible reason for the association in the extrapulmonary ARDS group could be that the HRCT score correlates with not only the degree of lung injury but also fibroproliferation changes. These findings suggest that the HRCT score is a very important prognostic factor for ARDS.
LDH is easily measured and used for biomarker of lung damage. Recently it was reported that LDH was independent predictor of 180-day mortality for acute exacerbation of idiopathic pulmonary fibrosis (22).
Collectively, the above findings suggest that the degree of lung damage and multiorgan failure may affect the prognosis in patients with ARDS, whether pulmonary or extrapulmonary. To our knowledge, this is the first report documenting HRCT and DIC scores as prognostic factors for pulmonary and extrapulmonary ARDS. Considering their importance, both factors should be evaluated in the treatment of ARDS, particularly that induced by infectious diseases.
The strengths of our study included the prospective collection of data as part of an ongoing ARDS study, incorporation of multiple potential confounding factors, performance of sensitivity analyses with different methods of confounding adjustment using standard regression, and use of propensity score-based IPTW.
This study also has some limitations. First, it was a single-center retrospective analysis of prospectively collected data, which could have biased the results. Moreover, multiple unmeasured variables may have affected the outcomes. However, these problems may be minimized relative to those encountered with a typical retrospective design. Second, it was a single-center study, and the patient number in the extrapulmonary ARDS group was relatively small. Therefore, we used methods such as IPTW to compensate for the small patient number. Third, the extended length of the study period may have improved patient outcomes based on supportive care that directly affected such outcomes, and could limit the validity of the study. However, over the past 20 years, there has been little evidence to support these suppositions. Fourth, it only included Japanese patients, and ethnic differences need to be considered. Fifth, we excluded patients with drug-associated ARDS. However, we recently reported that clinical characteristics and mortality differ between drug-associated ARDS and nondrug-associated ARDS (8). The results of the present study do not preclude the requirement for an etiology-focused analysis of ARDS in future studies. Moreover, ARDS was induced by infectious diseases in several patients in the present study. Therefore, we cannot generalize our results to all ARDS patients. Finally, the few cases of trauma, pancreatitis, and massive blood transfusion in the extrapulmonary ARDS group could have resulted in selection bias.
Conclusions
Our findings suggest that pulmonary and extrapulmonary ARDS may be comparable in terms of the prognosis and duration of ventilation, and that DIC and HRCT scores may be common clinical predictors of mortality with ARDS.
Acknowledgements
We would like to thank Hiroyuki Muranaka (Department of Total Quality Management, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Yasuhiro Gushima (Department of Emergency and Critical Care Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Norihiro Iwamoto (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Shigeo Hiroshige (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Makoto Takaki (Department of Emergency and Critical Care Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Mitsuko Honda (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Naoko Arakawa (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Aoi Teruya (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Yuko Yasuda (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Yoshitomo Eguchi (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Yoshihiko Sakata (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Naoki Shingu (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Jumpei Hisanaga (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), Tatsuya Nitawaki (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan), and Aiko Nakano (Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan) for their clinical assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutional review board (Approval number, 238). Informed consent was obtained from all individual participants included in the study.
References
- Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015;147:1539-48. [Crossref] [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Luo L, Shaver CM, Zhao Z, et al. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest 2017;151:755-63. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526-33. [PubMed]
- Ichikado K, Muranaka H, Gushima Y, et al. Fibroproliferative changes on high-resolution CT in the acute respiratory distress syndrome predict mortality and ventilator dependency: a prospective observational cohort study. BMJ Open 2012;2. [Crossref] [PubMed]
- Kawamura K, Ichikado K, Takaki M, et al. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: a retrospective study and propensity score analysis. Springerplus 2016;5:1193. [Crossref] [PubMed]
- Takaki M, Ichikado K, Kawamura K, et al. The negative effect of initial high-dose methylprednisolone and tapering regimen for acute respiratory distress syndrome: a retrospective propensity matched cohort study. Crit Care 2017;21:135. [Crossref] [PubMed]
- Anan K, Ichikado K, Kawamura K, et al. Clinical characteristics and prognosis of drug-associated acute respiratory distress syndrome compared with non-drug-associated acute respiratory distress syndrome: a single-centre retrospective study in Japan. BMJ Open 2017;7. [Crossref] [PubMed]
- Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- MacIntyre NR, Cook DJ, Ely EW Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001;120:375-95S. [Crossref] [PubMed]
- Ichikado K, Suga M, Muranaka H, et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: validation in 44 cases. Radiology 2006;238:321-9. [Crossref] [PubMed]
- Fujimoto K, Taniguchi H, Johkoh T, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol 2012;22:83-92. [Crossref] [PubMed]
- Gando S, Iba T, Eguchi Y, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med 2006;34:625-31. [Crossref] [PubMed]
- Walkey AJ, Wiener RS. Macrolide antibiotics and survival in patients with acute lung injury. Chest 2012;141:1153-9. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med 2002;30:2450-6. [Crossref] [PubMed]
- Hecker L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am J Physiol Lung Cell Mol Physiol 2018;314:L642-53. [Crossref] [PubMed]
- Agarwal R, Aggarwal AN, Gupta D, et al. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 2006;130:724-9. [Crossref] [PubMed]
- Agarwal R, Srinivas R, Nath A, et al. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? A meta-analysis. Chest 2008;133:1463-73. [Crossref] [PubMed]
- Sevransky JE, Martin GS, Mendez-Tellez P, et al. Pulmonary vs nonpulmonary sepsis and mortality in acute lung injury. Chest 2008;134:534-8. [Crossref] [PubMed]
- Gando S, Saitoh D, Ishikura H, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Crit Care 2013;17:R297. [Crossref] [PubMed]
- Sokai A, Tanizawa K, Handa T, et al. Asymmetry in acute exacerbation of idiopathic pulmonary fibrosis. ERJ Open Res 2017.3. [PubMed]