High resolution computed tomography findings in smear-negative pulmonary tuberculosis patients according to their culture status
Introduction
Tuberculosis (TB) is one the most common causes of death from an infectious disease worldwide, after Human Immunodeficiency Virus (HIV) infection, with an estimated 8.7 million new cases of TB in 2011 (1). Turkey is categorized as a low endemicity TB region according to the World Health Organization (2). Nearly one-half to one-third of the pulmonary tuberculosis (PTB) cases are smear-negative, and approximately 1.9 million new PTB cases were found to be smear-negative in 2011 (1). Smear negativity in PTB is a common clinical problem leading to serious delays in the establishment of a diagnosis. A significant proportion of the patients treated for smear-negative PTB never have bacteriological confirmation, even with improved culture methods. Therefore, most cases of smear-negative PTB are diagnosed on the basis of the clinical presentation, radiological findings and other laboratory tests. It is an important decision whether to treat a patient when the microbiological data does not support the clinical suspicion. Thus, we decided to examine the clinical features and high resolution computed tomography (HRCT) findings of smear-negative PTB patients at the beginning of antituberculosis treatment and to compare the findings of smear-negative, culture-positive PTB patients with smear-negative and culture-negative PTB patients.
Materials and methods
This was a retrospective cohort study conducted at the Pulmonary Department of Gulhane Military Medical Academy Haydarpasa Training Hospital. It was approved by the Ethics Review Board of our institution. Data of patients who were diagnosed with smear-negative PTB between January 2007 and December 2010 were reviewed. All the patients had at least three negative sputum smear reports for acid-fast bacillus (AFB) and/or two negative gastric lavage and/or bronchoalveolar lavage AFB reports. Mycobacterial cultures of all the specimens were performed on both Löwenstein-Jensen solid medium and in the BACTEC MGIT 960 liquid culture system (Becton, Dickinson Microbiology System, Sparks, Shannon, Ireland). A Gram stain and microorganism identification was performed in all the samples according to standard protocols to exclude pulmonary infections other than PTB. Initially all of the patients were treated with a single course of broad-spectrum antibiotics (excluding antituberculosis drugs and fluoroquinolones) in order to exclude pulmonary infections. Antituberculosis treatment was started if the radiologic findings and clinical parameters were consistent with active PTB. HRCTs were performed at the beginning of the treatment and at the third month of the treatment for follow-up. The patients who had negative cultures had a thorough follow-up clinical and radiological evaluation at the third month of therapy to determine whether there had been a response that could be attributed to the antituberculosis treatment.
The treatment was stopped in the patients who did not have radiological and clinical improvement by the third month. In these cases the radiological lesions were considered to be inactive or sequelae.
All the patients completed the six months course of antituberculosis therapy. No other etiology was defined. The patients were divided into two groups according to their culture results: smear-negative culture-positive and smear-negative culture-negative. All the patients were newly diagnosed smear-negative PTB cases without a previous history of TB. The standard 6-month antituberculosis treatment consisting of a 2-month initial phase of rifampicin, isoniazid, pyrazinamide and ethambutol, and a 4-month maintenance phase of rifampicin and isoniazid was given to the patients via directly observed treatment (DOTS).
Two radiologists, who were blinded to the culture and clinical results, evaluated the initial HRCT and interpreted the findings. If there was any disagreement between them, their final consensus report was recorded. The HRCT images were analyzed retrospectively for the morphology, number and lobar distribution of pulmonary lesions. The HRCT was performed using the Aquilion One dynamic volume CT (Toshiba, Japan) scanner, according to the Hi Rez Chest HCT (0.5 mm) imaging protocol: 0.5 s rotation time, pitch; fast, 120 kV, sure exposure standard mA, 0.5 mm ×64 scan slice thickness. The descriptive terms used to interpret the HRCT findings are as follows (3): micronodule (discrete, small, round, focal opacity less than 3 mm), large nodule (rounded or irregular opacity, well or poorly defined, measuring 3 mm to 3 cm in diameter), centrilobular nodule, tree-in-bud pattern, cavity, ground glass opacity, calcified nodule, consolidation, mass (>3 cm), interlobular septal thickening, bronchial wall thickening, bronchiectasis, emphysema, mediastinal lymphadenopathy (LAP, >10 mm), bulla, hilar LAP, calcified LAP and pleural thickening.
Number Cruncher Statistical Systems software (NCSS, East Kaysville, Utah, USA) was used for the statistical analysis in this study. The data are expressed as the means ± standard deviation (SD) for continuous data such as age. An independent t-test was used to compare differences between the groups for the continuous data. Pearson’s chi-square test and Fisher’s exact test were used to compare the distribution of the categorical data between the groups. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A P value <0.05 was considered statistically significant.
Results
There were a total of 85 patients. Seven patients were excluded from the study as they did not have radiological and clinical improvement after three months of the treatment, leaving 78 patients. There were 50 culture-positive patients (Group 1) and 28 culture-negative patients (Group 2). The patients (n=7) who had both a positive AFB culture and a histopathological diagnosis (caseation granulomatous inflammation) with surgical biopsy or transbronchial lung biopsy were also included in Group 1. No patients in our study population were HIV positive.
The mean patient-age was 22.48±3.18 years (range, 20-36 years). All the patients were male because the study was performed in a military hospital. There was no statistically significant difference in the mean age between the two groups (P=0.793).
Cough was the most frequent symptom, being present in 37% of the patients, followed by chest pain in 32%, expectoration in 18%, hemoptysis in 10%, fever in 9% and weight loss in 8% of the patients. There were no statistically significant differences in symptoms between the two groups (P>0.05).
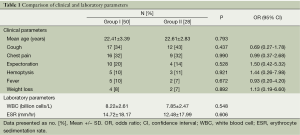
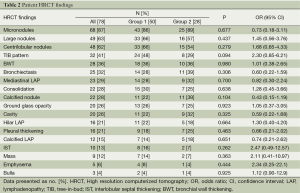
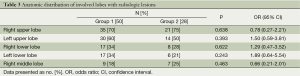
The clinical and laboratory data are presented in Table 1. There were no statistically significant differences in the laboratory parameters between the two groups (P>0.05). The most common HRCT findings were micronodules (87%), large nodules (63%), and centrilobular nodules (62%). The radiological data are presented in Table 2. Again, there were no significant differences in the radiological findings between the groups. The localization of the radiological lesions was also evaluated. Radiological lesions were observed in the right (72%) and left (56%) upper lobes most frequently (Table 3). There were no significant differences in the distribution of lesions between the two groups (P>0.05).
Full table
Full table
Full table
Discussion
The early diagnosis of PTB is critical for TB control. Patients with active smear-negative PTB are also capable of transmitting the infection (4). However, the diagnosis of a patient with smear-negative PTB is difficult and often delayed. In medical practice, only 68% of PTB are culture-confirmed, but the clinical features and HRCT findings of smear-negative and culture-negative PTB are not well known. Presently the most important criteria for establishing a presumptive diagnosis of smear negative PTB are based on clinical findings, radiographic signs, risk factors or a combination of these. In our study, we investigated the use of the clinical features and HRCT findings to differentiate smear-negative culture-positive PTB from smear-negative and culture-negative disease.
Cough was the most common symptom reported in both groups. Other symptoms were chest pain, expectoration, hemoptysis, fever and weight loss; Nakashi et al. also reported that cough was seen in 83% of patients as the most common symptom in smear-negative PTB (5). Chest pain is a symptom for PTB and is related to pleural involvement. This symptom was the second most common complaint in both groups. Interestingly, Tozkoparan et al. found chest pain to be the most common symptom (38%) for smear-negative PTB and emphasized the significantly high existence of chest pain in active smear-negative PTB (6). Other related symptoms in our patients were expectoration, hemoptysis, fever and weight loss. Our results showed that smear-negative culture-positive PTB cannot be discriminated from smear-negative and culture-negative PTB based on clinical symptoms and laboratory parameters.
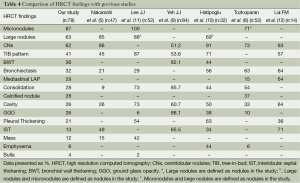
The chest radiograph as a tool for the diagnosis of active PTB is sensitive but limited by poor specificity (7). HRCT may identify a finding of PTB not seen on a chest radiograph (8). HRCT is usually recommended when the radiographic findings are normal or inconclusive, and PTB is suspected clinically, for the confirmation of diagnosis and determination of activity. Investigators have tried to design HRCT diagnostic criteria to rank the risk of PTB in patients with suspected PTB (5), and others have created an active PTB prediction model based on HRCT findings to differentiate smear-positive active PTB from other pulmonary infections (9). There are several studies about HRCT findings of smear-negative PTB and the role of HRCT in predicting the activity of PTB (6,8,10), but no previous studies have revealed the relationship between the HRCT findings and the AFB culture results. We found that micronodules, large nodules and centrilobular nodules were the most common HRCT findings in our study in both groups. The HRCT findings of smear-negative PTB were compared with previous studies in Table 4.
Full table
Micronodules were the most common HRCT finding of smear-negative PTB in our study. The four most common HRCT findings are presented in Figure 1. HRCT enables the creation of a differential diagnosis of diffuse micronodular lung disease by showing the distribution of micronodules in and around the secondary pulmonary lobule (13,14). HRCT findings of centrilobular nodules or a predominant distribution of the micronodules in the secondary lobule may help to distinguish bronchogenic spread from miliary PTB (15). We found that the presence of micronodules tended to be higher in Group 2, although there was no statistically significant difference between the groups. Micronodules are known as acute inflammatory lesions and are one of the radiological lesions showing active PTB (11). Lee et al. found that micronodules were present in all of the patients with newly diagnosed and bacteriogically proven PTB and were the most common HRCT finding seen in active PTB, as in our study (11). Micronodules are most often seen in the acute early stages of PTB and consist of solid caseous material within or around the terminal or respiratory bronchioles (16). Matsuoka et al. reported that computed tomography findings do not reliably discriminate between smear-negative patients and those with very few AFB excreting smear-positive patients, and the frequency of micronodules did not significantly differ among the four groups of smear-negative and smear- positive PTB (17). We suggest that micronodules appear to be a useful radiologic HRCT finding for both smear negative culture-positive and smear-negative culture-negative PTB.
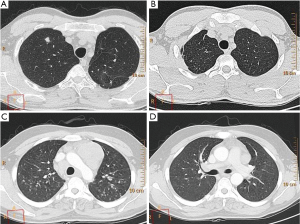
Centrilobular lesions on HRCT are highly sensitive and specific for active PTB (12). The presence of centrilobular nodules tended to be higher in Group 1 in our study, but there was no statistically significant difference between the two groups. Tozkoparan et al. showed that centrilobular nodules were the most common HRCT finding and were significantly more common in active smear-negative PTB (6). Hatipoglu et al. also found that centrilobular lesions or branching linear structures, with a tree-in-bud appearance and macronodules were most commonly seen in cases of active PTB (10). If there is a clinical suspicion, an HRCT can be used to show centrilobular lesions, which are the most common findings of early bronchogenic spread (10). Yeh et al. reported that the absence of centrilobular nodules (51.2%) is one of the five variables that are independent risk factors predictive of smear-positive, active PTB (9). Kosaka et al. determined that the frequency of centrilobular opacities did not differ between smear-negative and smear-positive PTB (18). We found large nodules in 63% of the patients with smear-negative PTB. Nakanishi et al. found that large nodules were seen in 85% of the patients with smear-negative culture-positive PTB, and large nodules were significantly associated with an increase in the risk for PTB (5). The presence of large nodules in Group 1 tended to be greater in our study, but there was no significant difference between the two groups.
We found a tree-in-bud pattern in 48% of the patients with smear-negative culture-positive PTB and in 29% of the patients with smear-negative culture-negative PTB. Tree-in-bud patterns on CT were first used by Im et al. for the endobronchial spread of PTB (16). The tree-in-bud pattern is thought to be a reliable criterion for active disease, but not pathognomonic for active PTB. Raniga et al. reported that the tree-in-bud pattern, suggestive of endobronchial spread, and hence active disease, was the most common and characteristic of the findings on HRCT (8). Although the smear-negative culture-positive group more frequently tended to have the tree-in-bud pattern, there was no significant difference in the tree-in-bud pattern between the two groups.
The radiologic findings of PTB occur in the apical or posterior segment of the upper lobes in the majority of adult patients and in the superior segment of the lower lobes in others (19). In our study, the HRCT findings were observed mainly in the right and left upper lobes. There was no significant difference in the lobar predominance (upper lobe vs. middle or lower lobes) between the groups. Okutan et al. also reported similar localizations on HRCT in those patients with smear-negative culture-negative PTB requiring a histopathological examination (20).
The main limitation in this study was the lack of HIV positive patients. Smear-negative PTB is more common among HIV positive patients (21). The HRCT findings of HIV positive patients with smear-negative PTB may be different or atypical (21,22). Other medical conditions may be misdiagnosed as smear-negative PTB and may complicate the clinical diagnosis of PTB in HIV positive patients (23). Reader experience is also another limitation in our study.
Conclusions
Significant differences were not observed in the HRCT findings and clinical features of smear-negative culture-positive and smear-negative culture-negative PTB patients. We conclude that it is not possible to predict those patients whose culture specimens will ultimately prove to be positive among those patients with smear-negative PTB according to their clinical features and HRCT findings. Additionally, the HRCT and clinical features were suboptimal for distinguishing smear-negative culture-positive PTB from smear-negative culture-negative PTB in this study. Thus, in cases of smear-negative and culture-negative PTB, the patient with compatible clinical and radiological features should be considered for tuberculosis treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 2012 WHO. Global Tuberculosis Report 2012. Available online: http://www.who.int/tb/publications/global_report/gtbr12_main.pdf
- Erdem H, Akova M. Leading infectious diseases problems in Turkey. Clin Microbiol Infect 2012;18:1056-67. [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [PubMed]
- Tostmann A, Kik SV, Kalisvaart NA, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis 2008;47:1135-42. [PubMed]
- Nakanishi M, Demura Y, Ameshima S, et al. Utility of high-resolution computed tomography for predicting risk of sputum smear-negative pulmonary tuberculosis. Eur J Radiol 2010;73:545-50. [PubMed]
- Tozkoparan E, Deniz O, Ciftci F, et al. The roles of HRCT and clinical parameters in assessing activity of suspected smear negative pulmonary tuberculosis. Arch Med Res 2005;36:166-70. [PubMed]
- Wang YH, Lin AS, Lai YF, et al. The high value of high-resolution computed tomography in predicting the activity of pulmonary tuberculosis. Int J Tuberc Lung Dis 2003;7:563-8. [PubMed]
- Raniga S, Parikh N, Arora A, et al. Is HRCT Reliable In Detecting Disease Activity In Pulmonary Tuberculosis? Indian J Radiol Imaging 2006;16:221-8.
- Yeh JJ, Yu JK, Teng WB, et al. High-resolution CT for identify patients with smear-positive, active pulmonary tuberculosis. Eur J Radiol 2012;81:195-201. [PubMed]
- Hatipoğlu ON, Osma E, Manisali M, et al. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax 1996;51:397-402. [PubMed]
- Lee JJ, Chong PY, Lin CB, et al. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol 2008;67:100-4. [PubMed]
- Lai FM, Liam CK, Paramsothy M, et al. The role of 67 gallium scintigraphy and high resolution computed tomography as predictors of disease activity in sputum smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis 1997;1:563-9. [PubMed]
- Lee KS, Kim TS, Han J, et al. Diffuse micronodular lung disease: HRCT and pathologic findings. J Comput Assist Tomogr 1999;23:99-106. [PubMed]
- Fujita J, Higa F, Tateyama M. Radiological findings of mycobacterial diseases. J Infect Chemother 2007;13:8-17. [PubMed]
- Fujita J, Bandoh S, Kubo A, et al. HRCT shows variations in appearance in disseminated tuberculosis in adults. Int J Tuberc Lung Dis 2006;10:222-6. [PubMed]
- Im JG, Itoh H, Shim YS, et al. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology 1993;186:653-60. [PubMed]
- Matsuoka S, Uchiyama K, Shima H, et al. Relationship between CT findings of pulmonary tuberculosis and the number of acid-fast bacilli on sputum smears. Clin Imaging 2004;28:119-23. [PubMed]
- Kosaka N, Sakai T, Uematsu H, et al. Specific high-resolution computed tomography findings associated with sputum smear-positive pulmonary tuberculosis. J Comput Assist Tomogr 2005;29:801-4. [PubMed]
- Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191:834-44. [PubMed]
- Okutan O, Tas D, Demirer E, et al. The clinical and radiological features of patients with smear and culture-negative pulmonary tuberculosis requiring histopathological examination. Turk Gogus Kalp Damar Cerrahısı Dergısı-Turkısh Journal of Thoracıc And Cardıovascular Surgery 2012;20:572-6.
- Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis 2003;3:288-96. [PubMed]
- Laissy JP, Cadi M, Boudiaf ZE, et al. Pulmonary tuberculosis: computed tomography and high-resolution computed tomography patterns in patients who are either HIV-negative or HIV-seropositive. J Thorac Imaging 1998;13:58-64. [PubMed]
- Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis 2000;4:97-107. [PubMed]


