Heart-lung transplantation: current indications, prognosis and specific considerations
Introduction
Over 25 years of experimentation were required to allow the first successful heart-lung transplantation (HLTx) in a human patient (1,2). In the late 1960s and early 1970s, HLTx was performed in 3 patients, all of whom died rapidly, the longest survival being 23 days. Then, in the early 1980s, a group in Stanford performed HLTx in 3 patients with pulmonary vascular disease, of whom 2 achieved long-term survivals (3). The number of HLTx procedures recorded in the registry maintained by the International Society of Heart and Lung Transplantation (ISHLT) peaked in 1989 then declined to less than 100 per year at present (4). This change is partly ascribable to the predominant initial use of HLTx in patients with pulmonary vascular disease or cystic fibrosis (CF), who are now generally managed by lung transplantation. The indications for HLTx continue to evolve. Currently, HLTx is considered the best option in selected patients who have end-stage heart and lung failure.
Indications
Complex congenital heart disease (CHD) complicated by Eisenmenger syndrome is the most common indication of HLTx (Table 1). HLTx is also indicated in patients who have both end-stage lung disease and either refractory left ventricular failure or objectively documented fibrosis or infarction of the right ventricle with right ventricular failure (6). The ISHLT registry has a total of 3,879 HLTxs performed in adults between January 1982 and June 2015 (4). The highest annual number of HLTxs in adults was 226, in 1989. The subsequent decline reflects advances in other treatments for pulmonary hypertension and heart failure combined with the use of isolated heart or lung transplantation in patients who would previously have been managed by HLTx. In the past decade, the annual number of HLTxs was only 49 to 92 worldwide (4). According to the ISHLT registry, the most common indication for HLTx from 1982 to 1991 was PAH, followed by CHD and CF (7). Nearly all patients who have PAH or CF are now managed with double-lung transplantation (DLTx) alone. Nevertheless, PAH accounted for as many as 27.3% of HLTx procedures performed between January 2004 and June 2015, compared to 35.5% for CHD (35%) and 11% for cardiomyopathy (11%) (4).
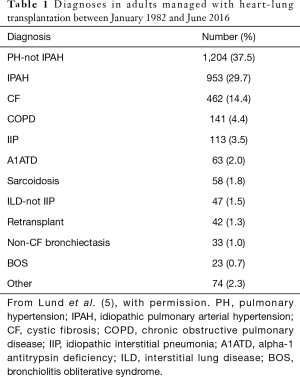
Full table
Candidates
Conditions for which HLTx is now viewed as the best transplant option include Eisenmenger syndrome complicating complex CHD, failed CHD repair, uncorrectable CHD, and severe left ventricular failure. As cardiomyopathy can result in right ventricular failure and early death, heart transplantation (HTx) may not be a good option when the right heart catheterization shows pulmonary vascular resistance (PVR) values above >3 Wood units or a transpulmonary pressure gradient above 15 mmHg (8). However, isolated HTx is usually considered an acceptable option in patients whose PVR falls below 4 Wood units (320 dyn·s/cm5) with treatment. In contrast, HLTx may be an alternative to HTx in patients whose PVR remains high despite treatment.
Uncertainty long prevailed regarding the best transplantation option in patients with PAH. However, in several studies, patients with PAH had similar outcomes after DLTx as after HLTx, even when they had right ventricular failure (9,10). After DLTx, PVR may return to normal, and acute or chronic right ventricular failure complicating severe pulmonary hypertension may resolve. As long as the left ventricle is normal, HLTx is not required, and DLTx should be considered the procedure of choice in virtually all patients with PAH (11). A 2015 study of patients in the Scientific Registry of Transplant Recipients who had right ventricular failure complicating idiopathic PAH demonstrated similar long-term survival between HLTx and DLTx recipients (10).
However, transplant centers vary widely regarding the degree of right and left ventricular function impairment deemed consistent with DLTx. Cutoffs below which DLTx is deemed inappropriate vary between 10% and 25% for the right ventricular ejection fraction and between 32% and 50% for the left ventricular ejection fraction (LVEF) (12-14). According to one group, an LVEF of 30% to 35% may be compatible with DLTx provided the cardiac index is above 2.2 L/min/m2 and the pulmonary capillary wedge pressure or left ventricular end-diastolic pressure is no greater than 15 mmHg, as assessed by right heart catheterization (11).
Patients with end-stage lung disease and reparable heart abnormalities may be eligible for DLTx combined with the appropriate cardiac procedure (e.g., reparative CHD surgery, coronary artery bypass grafting, or surgery to repair or replace a valve) (15). Waiting times are shorter and the likelihood of receiving a transplant higher with DLTx than with HLTx. Transplant allocation practices in North America and Europe have made it extremely difficult to obtain heart-lung blocks (16,17).
Extracorporeal membrane oxygenation (ECMO) as a bridge to DLTx is now a recognized treatment option in patients with end-stage lung disease. However, data on ECMO in patients awaiting transplants are limited, and the potential benefits of ECMO as a bridge to HLTx in adults remain unclear (18,19). Data from small observational cohorts suggest that ECMO bridging is safe and provides good short-term outcomes (11). However, data on HLTx recipients recorded in the United Network for Organ Sharing (UNOS) database between 1995 and 2011 show poorer survival in patients who required pretransplant invasive mechanical ventilation or ECMO than in similar controls: 1-month survival was 20% in the ECMO group and 83.5% in the control group, and corresponding values for 5-year survival were 20% and 45.4% (20).
Increased pretransplant mortality risk among HLTx candidates
Among HLTx candidates in France in 2011–2016, within 12 months after listing, only 33% received transplants and as many as 32% were taken off the list because of death or severe clinical deterioration (17). Factors explaining the decreased use of HLTx may include diminished availability due to allocation practices, HLA antibody development due to blood transfusions, and shorter height of patients with CHD (21). Most HLTx candidates have a history of cardiovascular surgery, which results in the development of adhesions, anatomical alterations, and collateral blood vessels, thereby creating technical difficulties (22). HLTx requires cardiopulmonary bypass, which carries a risk of complications including coagulation disorders, decreased brain perfusion, acute kidney injury, and arrhythmias (22). Consequently, either HTX or DLTx deserves preference if allowed by the patient’s condition.
Time of listing
According to general recommendations, transplantation should be considered when optimal medical therapy is expected to provide no more than 2 years’ survival and there are no contraindications (6). However, debate continues to surround the best time for HLTx. Indeed, in patients with Eisenmenger syndrome, in whom HLTx is the preferred procedure, long-term survival is unpredictable and patient-specific. In addition, referral for HLTx is in order in patients with worsening ventricular failure and pulmonary hypertension. Factors that govern listing decisions include cardiac anatomy, hemodynamic status, and the general condition of the patient. Another criterion for transplant candidacy evaluation is a decline in quality of life related to worsening cardiopulmonary failure with increasingly frequent hospital admissions. Listing criteria include New York Heart Association (NYHA) class III or IV right ventricular failure in a patient receiving optimal medical treatment, with a cardiac index below 2 L/min/m2 and right atrial pressure above 15 mmHg (6). Conditions that may require earlier listing include documented or possible pulmonary veno-occlusive disease (PVOD) and pulmonary capillary hemangiomatosis with significant hemoptysis (6).
Postoperative management and complications
Postoperative care is the same after HLTx as after DLTx or single-lung transplantation (SLTx). Indeed, the lungs, and not the heart, are at the origin of most postoperative complications, including infections and acute and chronic rejection (21).
The induced immunosuppression after HLTx is comparable to that after DLTx or SLTx, i.e., usually more profound than after HTx. The follow-up of HLTx recipients includes closely-spaced lung function tests, chest radiographs, and outpatient assessments. The optimal interval between surveillance bronchoscopies after HLTx is not agreed on, and most centers usually apply their protocol designed for lung transplant recipients. Protocols for aspirin, beta-blocker, and statin therapy in HLTx recipients vary across centers, reflecting the absence of clear guidelines.
Acute cellular rejection in either the heart or lungs after HLTx is less common than after isolated heart or lung transplantation (23). Of note, acute cellular rejection involves the lungs more often than the heart (23). Acute cellular rejection of the heart may be synchronous or asynchronous with lung rejection but is uncommon and usually occurs early (23). Surveillance endomyocardial biopsies, although obtained routinely after HTx to look for asymptomatic rejection, is rarely performed after HLTx, particularly beyond 4 to 6 months (24). In addition, there is some recent evidence that using echocardiography alone to monitor heart allograft function is associated with similar outcomes to those seen with routine endomyocardial biopsies (25).
Most infectious complications after HLTx involve the lungs and are comparable in frequency to those recorded after DLTx or SLTx. In HLTx recipients, the curative and prophylactic treatment of infectious complications is no different than after lung transplantation.
Long-term outcomes are comparable after HLTx and after DLTx/SLTx, i.e., significantly worse than after HTx. Most complications in HLTx recipients involve the lungs (21). HLTx may be followed by chronic rejection of the heart, the lungs, or both. Coronary artery vasculopathy (CAV) occurs less often than bronchiolitis obliterans syndrome (BOS) after HLTx. Thus, in one study, rates of CAV 1, 3, 5, and 10 years after HLTx were 3%, 7%, 9%, and 27%, respectively, compared to 8%, 27%, 42%, and 62% for BOS (26).
Survival
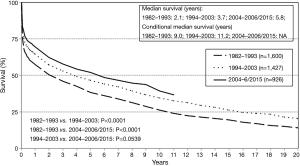
Since the first HLTx procedures in the 1980s, survival has improved steadily. The outcomes of 23 HLTx procedures performed in 22 patients during the first 3.5 years of the Stanford program were reported in 1985 (27). The diagnoses were Eisenmenger syndrome with CHD in 12 patients and idiopathic PAH in 10 patients. Of the 22 patients, 6 (27%) died within 30 days or before hospital discharge. Predicted actuarial survival was 71% after 1 year and 57% after 2 years (27). The ISHLT registry has data for 1,216 patients transplanted in 1982–1991, whose outcomes were similar, with 25.4% early mortality and survival rates at 1, 2, 5, and 10 years of 56%, 49%, 37.7%, and 26%, respectively (28). In contrast, the 2016 ISHLT report for 2004–2014 indicates far higher survival rates of 63%, 52%, 45%, and 32% after 1, 2, 5, and 10 years, respectively, and a median survival of 5.8 years (26) (Figure 1). Although long-term survival has improved, the most substantial gains have been achieved during the early posttransplant period. In recipients transplanted in 2004–2014 who were alive after 1 year, median survival was longer than 10 years (26). Factors that explain these survival gains include improved selection of patients to HLTx, advances in surgical technique, the development of better immunosuppressive regimens for preventing rejection, and new insights into the factors associated with morbidity and mortality.
Causes and predictors of mortality
Survival after HLTx has improved over time, although the causes of death have remained unchanged. During the first month after HLTx, the main causes of death are posttransplant graft failure, technical complications, and infection, whereas BOS and chronic lung allograft dysfunction cause the most deaths beyond the first year. Mechanical ventilation or circulatory support before HLTx is associated with poorer outcomes. Thus, an analysis of the UNOS database for HLTx procedures done in 1995–2011 showed lower survival among patients who required pre-transplant ventilation or ECMO as in controls similar for recipient and donor age and gender and for ischemic time, both during the first month (20% with vs. 83.5% without ECMO) and after 5 years (20% vs. 45.4%, P<0.0001) (20). These findings indicate a marked difference in outcomes depending on pre-HLTx severity. Due to the small number of HLTx procedures performed each year, studies of risk factors for death are scarce and produced limited findings. A 2016 study of ISHLT data found associations linking higher mortality to older donor age and to HLTx for conditions other than idiopathic PAH. Patients managed at centers with low HLTx volumes did not have higher mortality, but volume was low in all centers (28).
Long-term immunosuppressive therapy is the most common source of morbidity after HLTx. Among HLTx recipients entered in the ISHLT registry in 1994–2013, 88.1% had hypertension and 70% hyperlipidemia 5 years after transplantation. Furthermore, 45.5% had kidney dysfunction, which required dialysis in 2.1% and kidney transplantation in 1.1%. Consistent with the statement above that early complications usually involve the lungs, BOS occurred in 28.7% of patients, compared to 8.2% for CAV. Retransplantation was uncommon, with only 57 HLTx recipients undergoing a second HLTx from January 1982 through June 2015 (26). The finding of poorer outcomes after repeat HLTx than after primary HLTx warrants caution when considering a second HLTx procedure.
Benefits of HLTx depending on the indication
Pulmonary hypertension with right heart failure
Outcomes of SLTx, DLTx, and HLTx done in 1989–1993 in 30 patients with Eisenmenger syndrome and 27 with primary pulmonary hypertension were assessed retrospectively (12). Indications for HLTx were LVEF below 35%, significant coronary artery disease, and Eisenmenger syndrome complicating complex CHD. Mortality after 1 to 3 months was similar in these three groups. However, the SLTx recipients had lower 1-year survival (38%) and a significantly higher incidence of graft failure (SLTx, 82%; DLTx, 59%; and HLTx, 33%; P<0.05). In a retrospective comparison of outcomes of HLTx (n=157) and DLTx (n=67) performed in 1986–2008, our group found no differences in 1-, 5-, 10-, or 15-year survival (HLTx: 70%, 50%, 39%, and 26% vs. DLTx, 79%, 52%, 43%, and 30%, respectively; P=0.46), despite more severe pretransplant disease in the HLTx group (with greater severity of right ventricular, liver, and kidney dysfunction and higher inotropic support requirements; P<0.05 for each) (9). The proportion of patients free of BOS after 10 years was significantly higher after HLTx than after DLTx (79% vs. 74%, P=0.035). In a Scientific Registry of Transplant Recipients study of HLTx and DLTx procedures performed in 1987–2012, the comparison of 261 HLTx patients and 667 DLTx patients showed no difference in overall survival (10). However, patients who required ICU admission before transplantation had a significantly better 8-year survival rate after HLTx than after DLTx (40% vs. 20%, P=0.043). Thus, patients with severe right heart failure may fare better overall with HLTx than with lung transplantation alone.
CHD
About 75% to 85% of patients with CHD survive to adulthood, and about 10% may then eventually require transplantation at some point (29). Decisions about whether and when to transplant incorporate many factors, including the type of defect, severity of the disease, and availability of organs.
Recent studies have identified markers of poor outcome in large patient cohorts with CHD (30,31). First, in selected patients with reparable CHD, same-stage LTx may constitute an alternative to HLTx. A retrospective study compared outcomes after DLTx with prior or same-stage CHD repair (n=35) and after HLTx (n=16) performed in pediatric patients in 1990–2003 (15). The most common diagnoses in the DLTx and repair group were ventricular septal defect (VSD), pulmonary venous obstruction, and pulmonary atresia. The two groups were similar for 1-, 3-, and 5-year survival (HLTx: 66.5%, 66.5%, and 60%; and DLTx, 62.9%, 51.4%, and 51.4%; respectively; P=0.852) and for survival without BOS (HLTx: 77.8%, 51.9%, and 38.9%; and DLTx: 72.9%, 54.7%, and 54.7%; respectively; P=0.442). In this population, HLTx was the preferred option in patients with multiple and complex CHD, and DLTx was considered only when the expected ischemic time needed for CHD repair was no greater than 60 min. Other studies demonstrated better outcomes after HLTx than after DLTx or SLTx with or without CHD repair in patients with Eisenmenger syndrome. A study of UNOS and ISHLT registry data compared long-term outcomes of HLTx (n=430), DLTx (n=106), and SLTx (n=69) in patients with CHD and Eisenmenger syndrome (32). By multivariable analysis, survival was significantly better with HLTx than with lung transplantation in patients with VSD (HLTx for VSD vs. atrial septal defect or patent ductus arteriosus: risk ratio, 0.517; P=0.0001; lung transplantation vs. HLTx for VSD, risk ratio, 1.817; P=0.035), and HLTx provided a significantly higher 1-year survival rate in the groups with VSD (71.4%) and multiple congenital anomalies (77.6%, P=0.011) (33). Other studies found no significant differences in HLTx outcomes in patients with vs. without Eisenmenger syndrome and suggested that HLTx was effective and safe in both groups (29,32,34-37).
Conclusions
Although in numerical decline for several decades, HLTx is currently viewed as the best procedure in carefully selected patients. Indeed, HLTx offers better survival to patients who have severe heart failure or complex CHD complicated with pulmonary hypertension. Furthermore, HLTx is associated with a usually lower ischemic time, producing better outcomes; a lower rate of BOS-related mortality; and significantly better postoperative cardiac function.
Compared to DLTx, HLTx does not produce worse outcomes when used to treat CHD or idiopathic PAH. Nonetheless, HLTx should be reserved for those patients with no other therapeutic options. In patients with Eisenmenger syndrome, the optimal timing of HLTx varies widely across patients. However, listing patients before their disease becomes so severe as to preclude good posttransplant outcomes is crucial.
The management of these patients with complex needs requires a multidisciplinary model. Close monitoring for declining function is imperative to ensure that patients are considered for HLTx listing in a timely manner. In patients with PAH, DLTx is the preferred treatment when heart function remains acceptable, whereas HLTx may need to be considered in the event of severe right and/or left heart failure.
Acknowledgements
Mrs. Antoinette Wolfe for editing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Webb WR, Howard HS. Cardio-pulmonary transplantation. Surg Forum 1957;8:313-7. [PubMed]
- Lower RR, Stofer RC, Hurley EJ, Shumway NE. Complete homograft replacement of the heart and both lungs. Surgery 1961;50:842-5. [PubMed]
- Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982;306:557-64. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1264-77. [Crossref] [PubMed]
- Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037-46. [Crossref] [PubMed]
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1-15. [Crossref] [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [Crossref] [PubMed]
- Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016;35:1-23. [Crossref] [PubMed]
- Fadel E, Mercier O, Mussot S, et al. Long-term outcome of double-lung and heart-lung transplantation for pulmonary hypertension: a comparative retrospective study of 219 patients. Eur J Cardiothorac Surg 2010;38:277-84. [Crossref] [PubMed]
- Hill C, Maxwell B, Boulate D, et al. Heart-lung vs. double-lung transplantation for idiopathic pulmonary arterial hypertension. Clin Transplant 2015;29:1067-75. [Crossref] [PubMed]
- Toyoda Y, Toyoda Y. Heart-lung transplantation: adult indications and outcomes. J Thorac Dis 2014;6:1138-42. [PubMed]
- Bando K, Armitage JM, Paradis IL, et al. Indications for and results of single, bilateral, and heart-lung transplantation for pulmonary hypertension. J Thorac Cardiovasc Surg 1994;108:1056-65. [PubMed]
- Olland A, Falcoz PE, Canuet M, et al. Should we perform bilateral-lung or heart–lung transplantation for patients with pulmonary hypertension? Interact Cardiovasc Thorac Surg 2013;17:166-70. [Crossref] [PubMed]
- Pielsticker EJ, Martinez FJ, Rubenfire M. Lung and heart-lung transplant practice patterns in pulmonary hypertension centers. J Heart Lung Transplant 2001;20:1297-304. [Crossref] [PubMed]
- Choong CK, Sweet SC, Guthrie TJ, et al. Repair of congenital heart lesions combined with lung transplantation for the treatment of severe pulmonary hypertension: a 13-year experience. J Thorac Cardiovasc Surg 2005;129:661-9. [Crossref] [PubMed]
- Colvin-Adams M, Valapour M, Hertz M, et al. Lung and heart allocation in the United States. Am J Transplant 2012;12:3213-34. [Crossref] [PubMed]
- Agence de la biomédecine - Le rapport annuel médical et scientifique 2016. Available online: https://www.agence-biomedecine.fr/annexes/bilan2016/donnees/organes/04-coeur-poumon/synthese.htm
- Strueber M, Hoeper MM, Fischer S, et al. Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device. Am J Transplant 2009;9:853-7. [Crossref] [PubMed]
- Gregoric ID, Chandra D, Myers TJ, et al. Extracorporeal membrane oxygenation as a bridge to emergency heart-lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2008;27:466-8. [Crossref] [PubMed]
- Jayarajan SN, Taghavi S, Komaroff E, et al. Impact of extracorporeal membrane oxygenation or mechanical ventilation as bridge to combined heart-lung transplantation on short-term and long-term survival. Transplantation 2014;97:111-5. [Crossref] [PubMed]
- Idrees JJ, Pettersson GB. State of the Art of Combined Heart-Lung Transplantation for Advanced Cardiac and Pulmonary Dysfunction. Curr Cardiol Rep 2016;18:36. [Crossref] [PubMed]
- Pasupneti S, Dhillon G, Reitz B, et al. Combined Heart Lung Transplantation: An Updated Review of the Current Literature. Transplantation 2017;101:2297-302. [Crossref] [PubMed]
- Pinderski LJ, Kirklin JK, McGiffin D, et al. Multi-organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant 2005;24:1828-33. [Crossref] [PubMed]
- Glanville AR, Imoto E, Baldwin JC, et al. The role of right ventricular endomyocardial biopsy in the long-term management of heart-lung transplant recipients. J Heart Transplant 1987;6:357-61. [PubMed]
- Technology CM. 829 - Non Invasive Monitoring of Acute Allograft Rejection in Heart Transplantation: Long-term Outcomes of the “No Biopsy Approach.” Available online: https://cslide-us.ctimeetingtech.com/ishlt2018/attendee/eposter/poster/819
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Dawkins KD, Jamieson SW, Hunt SA, et al. Long-term results, hemodynamics, and complications after combined heart and lung transplantation. Circulation 1985;71:919-26. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Hosseinpour AR, Cullen S, Tsang VT. Transplantation for adults with congenital heart disease. Eur J Cardiothorac Surg 2006;30:508-14. [Crossref] [PubMed]
- Kempny A, Hjortshøj CS, Gu H, et al. Predictors of Death in Contemporary Adult Patients With Eisenmenger Syndrome: A Multicenter Study. Circulation 2017;135:1432-40. [Crossref] [PubMed]
- Hjortshøj CMS, Kempny A, Jensen AS, et al. Past and current cause-specific mortality in Eisenmenger syndrome. Eur Heart J 2017;38:2060-7. [Crossref] [PubMed]
- Stoica SC, McNeil KD, Perreas K, et al. Heart-lung transplantation for Eisenmenger syndrome: early and long-term results. Ann Thorac Surg 2001;72:1887-91. [Crossref] [PubMed]
- Waddell TK, Bennett L, Kennedy R, et al. Heart-lung or lung transplantation for Eisenmenger syndrome. J Heart Lung Transplant 2002;21:731-7. [Crossref] [PubMed]
- Stoica SC, Perreas K, Sharples LD, et al. Heart-lung transplantation for Eisenmenger’s syndrome: operative risks and late outcomes of 51 consecutive cases from a single institution. J Heart Lung Transplant 2001;20:173-4. [Crossref] [PubMed]
- Hopkins WE, Ochoa LL, Richardson GW, et al. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant 1996;15:100-5. [PubMed]
- Goerler H, Simon A, Gohrbandt B, et al. Heart-lung and lung transplantation in grown-up congenital heart disease: long-term single centre experience. Eur J Cardiothorac Surg 2007;32:926-31. [Crossref] [PubMed]
- Vouhé PR, Tamisier D, Le Bidois J, et al. Pediatric cardiac transplantation for congenital heart defects: surgical considerations and results. Ann Thorac Surg 1993;56:1239-47. [Crossref] [PubMed]


