Relation between inflammatory cytokine levels in serum and bronchoalveolar lavage fluid and gene polymorphism in young adult patients with bronchiectasis
Introduction
While bronchiectasis was a disease of all ages before the use of antibiotics, today it is a disease that is most commonly seen in childhood. As a result of obstruction of the bronchi due to any hereditary or acquired cause, colonized microorganisms in that region and inflammation cause permanent destruction in the walls of the bronchi and as a result, bronchiectasis develops (1-3).
In studies that were performed to research cytokine gene polymorphism in chronic inflammatory diseases, it was seen that there is a correlation between cytokine levels and various cytokine gene variants (4). As the presence of cytokine gene polymorphism may affect the cytokine levels, we believe that such a polymorphism may be present in bronchiectasis in which there is chronic inflammation.
This study was designed to determine the levels of pro-inflammatory and anti-inflammatory cytokines, which are the inflammatory markers, in serum and bronchoalveolar lavage (BAL) fluid of patients with bronchiectasis and to investigate the presence or absence gene polymorphism of these cytokines or not.
Material and methods
This study was conducted in the GATA Haydarpasa Training Hospital, Chest Disease Service between June 2011 and June 2012 in a prospective and controlled manner. Approval was obtained from the hospital ethics committee.
Study population
Young adult patients who were admitted to the Chest Disease Service with the diagnosis of bronchiectasis during the study period constituted the study population. The individuals that did not meet the study criteria (the patients who had previously had tuberculosis, patients diagnosed with cystic fibrosis, the patients diagnosed with immune deficiency, patients and the controls below the age of 20 years, individuals that could not tolerate the bronchoscopy procedure, those that had contraindications for the procedure, and patients that the diagnosis of bronchiectasis could not be made), as well as the individuals that did not sign the written consent were not included the study. All patients with bronchiectasis were included in the study after at least two months of follow-up.
Sixty cases with bronchiectasis were included the study; however eight cases were excluded from the study because these cases were accepted as pseudobronchiectasis, as they possessed the criteria for lung infection and their bronchiectasis recovered on high resolution computed tomography during control. Six cases were excluded from the study as they did not accept the bronchoscopy procedure. Two cases were excluded from the study as they did not sign the informed consent form. Four cases were excluded from the study as the test results could not be found in the automatization system. A total of 40 patients with stable disease, who were found to be eligible for the study and who provided written informed consent, were included in the study.
Twenty individuals with the same age and gender in the study group, who had an indication for a bronchoscopy procedure (the cases in which the etiology of hemoptysis, dyspnea, and chronic cough were selected) and in which no diseases were detected in the laboratory examinations and from whom written informed consent was obtained, were accepted as the control group.
Obtaining study samples
After informing the patients that had no contraindication for bronchoscopy about the procedure, written informed consent was obtained. Following topical anesthesia, bronchoscopy was performed with a fiberoptic bronchoscopy device through the oral route to the study group of 40 individuals and to the control group of 20 individuals who had bronchoscopy indication. The samples of BAL fluid that were taken from the case and the control groups during the procedure and the serum samples of the cases that were taken during admission were stored at –80 °C.
Cytokine levels
By using commercially available kits, the cytokine levels [interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α] were studied in serum and BAL fluid samples that were taken from the case and the control groups and the levels were determined. (Assaypro trademark, Catalogue Number: EI1006-1, Missouri, United States of America). The ELISA method was used in the measurements of serum and BAL fluid cytokine levels.
DNA extraction and single nucleotide polymorphism
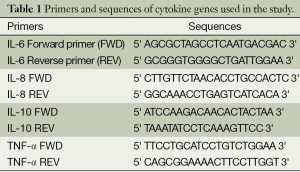
Using a NucleoSpin blood (Mavherey-Nagel) extraction kit, DNA was isolated from the leucocytes in the blood that were obtained for complete blood count during the admission of patient and the control groups. The Sanger method was used for DNA sequence analysis in investigation of the single nucleotide polymorphisms (SNP) in gene regions that are responsible for cytokine secretion (5). The forward and reverse primers that were used to determine polymorphisms were achieved in commercially available lyophilized forms (Invitrogen Life Technologies, USA). Primers and sequences of IL-6, IL-8, IL-10 and TNF-α genes used in our study are given in Table 1. A total of eight polymorphisms, one at the -174 G/C promoter region of IL-6 gene, one at the -251 A/T, the -161 C/A promoter region of the IL-8 gene, one at the -1082 G/A, -819 C/T, the -592 C/A promoter region of the IL-10 gene, one at -308 G/A, and -238 G/A promoter region of the TNF-α gene were detected by SNP genotyping polymerase chain reaction (PCR) and automatic DNA sequence analysis. The genotypes of the IL-6 -174 G/C, IL-8 -251 A/T, IL-10 -592 C/A and TNF-α -308 G/A polymorphism by DNA sequence analysis are demonstrated in Figure 1.
Full table
Statistics
SPSS 13.0 program was used for statistical analysis and the significance was evaluated at the level of P<0.05. During the evaluation of the study data, in addition to the descriptive statistical methods, independent samples t-test was used for normal distribution variables and the Mann-Whitney U-test was used for the variables that were not normally distributed. Logistic regression analysis was used for evaluation of gene polymorphism. The chi-square test was used in comparison of genotype and allele frequency ratios and odds ratio (relative risk) with a confidence interval of 95% was calculated to determine risk factors.
Results
This study was conducted on a total of 60 subjects who were comprised of 40 patients with clinically stable bronchiectasis and 20 control subjects. The study was conducted in a military hospital. The males were the dominant gender in the study due to the institutional characteristics of a military hospital. Therefore, all subjects included in the study and control groups were comprised of males. Of the cases, 12 were diagnosed in other centers before admission to the military hospital, and 29 patients were recently diagnosed with bronchiectasis.
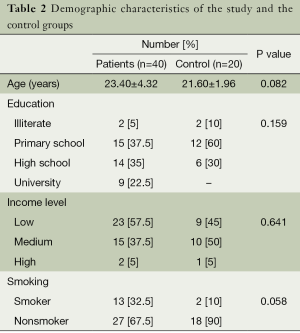
The mean age of the study group was 23.04±4.62 and the mean age of the control group was 23.04±2.62. There was no statistical difference between the study and the control groups in terms of age (P=0.82). The demographic characteristics of the study and the control groups are presented in Table 2.
Full table
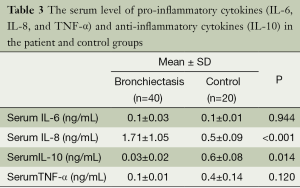
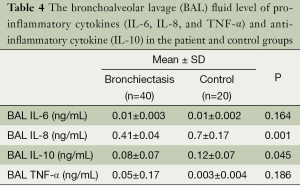
The serum and BAL fluid levels of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α) and anti-inflammatory cytokine (IL-10) were determined in the case and control groups.
No statistically significant difference was detected between groups in terms of serum IL-6 and TNF-α levels (P=0.944 and P=0.120). The serum IL-8 level of the study group was higher than the control group and this difference was statistically significant (P=0.001). The serum IL-10 level of the study group was lower than the control group and this difference was statistically significant (P=0.014) (Table 3).
Full table
There was no statistically significant difference between groups in terms of IL-6 and TNF-α levels in BAL fluid (P=0.164 and P=0.186). The IL-8 level in the BAL fluid in the study group was higher than the control group (P=0.001) and the IL-10 level of the study group was lower (P=0.045) (Table 4).
Full table
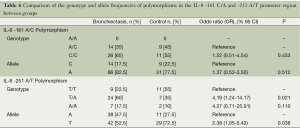
Allele and genotype analysis of IL-6 -174 G/C, IL-8 -161 C/A, IL-8 -251 A/T, IL-10 -592 C/A, IL-10 -819 C/T, IL-10 -1082 G/A, and TNF-α -308 G/A, TNF-α 238 G/A polymorphism was conducted on 60 samples taken from the case and control groups.
When the allele and genotype frequency of the IL-6 -174 G/C polymorphism was examined, no significant difference was found between the groups in terms of G and C alleles, and CC, GC, and GG, genotype frequencies, and the disease risk (P=0.174, P=0.34, P=0.88, respectively) (Table 5).

Full table
When the allele and genotype frequency of the IL-8 -161 C/A polymorphism was examined, no significant difference was found between groups in terms of A and C allele frequencies and AC and CC genotypes and disease risk (P=0.512, P=0.453, respectively). When the allele and genotype frequency of IL-8 -251 A/T polymorphism was examined, a significant difference was found between two groups in terms of allele frequencies (P=0,038). The T allele increased the risk by 2.38 fold according to A and this risk was statistically significant (OR =2.38, 95% CI =1.05-5.42, P=0.038). The AA genotype increased the risk by 4.27 fold according to TT (OR =4.27; 95% CI =0.71-25.91, P=0.11), and a significant difference was detected between the two groups in terms of TA and TT genotypes (P=0.021). The TA genotype increased the risk by 4.19 fold according to TT (OR =4.19; 95% CI =1.24-14.17; P=0.021) and this risk was statistically significant (Table 6).
Full table
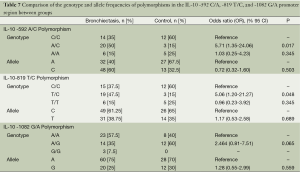
In the IL-10 -592 C/A polymorphism, there was no significant difference in the study group in terms of A and C allele frequencies and no significant difference between the two groups in terms of AA and CC genotypes and disease risk (P=0.503, P=0.345, respectively). There was a significant difference between the two groups in terms of AC genotype numbers, and the AC genotype frequency was significantly higher in the study group (P=0.017). The genotype AC increased the risk by 5.71 fold according to CC (OR =5.71, 95% CI =1.35-24.06, P=0.017) and this risk was statistically significant. In the IL-10 -819 T/C polymorphism, no significant difference was detected between the two groups in terms of T and C allele frequencies and TT and CC genotypes and disease risk (P=0.689, P=0.345, respectively). The TC genotype frequency was significantly higher in the study group and this was statistically significant (P=0.048). The TC genotype increased the risk by 5.06 fold according to the CC genotype (OR =5.06; 95% CI =1.20-21.27, P=0.048) and this risk was statistically significant. In the IL-10 -1082 G/A polymorphism, there was no significant difference between the two groups in terms of A and G allele frequencies, and GA and AA genotypes and disease risk (P=0.559, P=0.065, respectively) (Table 7).
Full table
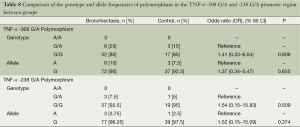
In the TNF-α -308 G/A polymorphism, no significant difference was detected between the groups in terms of A and G allele frequencies, and GA and GG genotypes and disease risks (P=0.655, P=0.806, respectively). In the TNF-α -238 G/A polymorphism, there was no significant difference between groups in terms of A, G allele frequencies, GA and GG genotypes and disease risk (P=0.374, P=0.509, respectively) (Table 8).
Full table
Discussion
In this study, the inflammatory cytokine levels, which are markers of inflammation, and the gene polymorphism of these cytokines, were investigated in cases with bronchiectasis. It was observed that the IL-8 and IL-10 levels in serum and BAL were statistically different in bronchiectasis, and gene polymorphism was related to these cytokines.
Olveira et al. reported a relationship between IL-6 and TNF-α and respiratory parameters in patients with bronchiectasis. In their study, serum levels of IL-6 were significantly higher in patients with bronchiectasis when compared to the levels in the control group. However, the TNF-α level was not different between the two groups (6). In the current study, although levels of IL-6, a pro-inflammatory cytokine, in serum and BAL was found to be higher in the study group; when compared to the control group a statistically significant difference was not detected. Also high levels of serum IL-8 in the patients and the high levels of IL-8 in BAL were parallel. This significance and correlation shows that the bronchial inflammation in patients who are not in the exacerbation period continues. This gives rise to the thought that if inflammation continues, the bronchial tissue injury will also continue at different localizations and with different intensities. When the serum and BAL fluid levels of TNF-α were examined, no statistically significant difference was found between the groups.
IL-8, which is chemo-attractive for neutrophils, can be found at high concentrations in induced sputum and BAL samples of patients with bronchiectasis and chronic obstructive lung disease (7). Guran et al. investigated the association between symptom scores, spirometry, high resolution computed tomography findings and inflammatory markers (TNF-α and IL-8 levels) in induced sputum in 27 children with stable non-cystic fibrosis bronchiectasis. They found that symptom scores correlated significantly with FEV1 and sputum IL-8 levels, and a significant correlation existed between HRCT severity scores and symptoms, FEV1, sputum IL-8 and TNF-α levels. In conclusion, the researchers concluded that the patients with bronchiectasis have an ongoing inflammatory process (8). TNF-α and IL-8 are strong chemo-attractive mediators and cause chemotaxis and activation of neutrophils. TNF-α is a strong paracrine and autocrine regulator that shows local effects in immunoinflammatory reactions at low concentrations. Studies show that TNF-α is the most important cytokine in acute inflammation and antitumor immunity. TNF-α regulates adhesion and chemotaxis by activating the neutrophils and endothelial cells (9,10).
Bergin et al. showed high levels of IL-8 in airway samples from patients with non-cystic fibrosis bronchiectasis. The finding of high IL-8 levels supports the use of appropriate anti-inflammatory therapies (11). Although the physiological importance of high IL-8 levels in the stable period of bronchiectasis cases is not known, it supports the fact that inflammation continues and the systemic cellular response is active in even stable periods. Thus, the thought of the importance and necessity of anti-inflammatory treatment in bronchiectasis emerges. The increased levels of various cytokines in plasma or serum of patients with bronchiectasis supports the thought that the local inflammatory response is connected to systemic circulation with these mediators. Thus, it can be assumed that the serum levels of inflammatory cytokines may be helpful in the detection of the severity and the progression of the disease. In the current study, while the serum levels of IL-6, IL-8, and TNF-α in the study group were higher than the control group, only the difference in the IL-8 levels was statistically significant. There was no statistically significant correlation between IL-6 and TNF-α levels and bronchiectasis. In one study, the serum levels of TNF-α were significantly higher in patients with bronchiectasis when compared to the control group. However, IL-8 levels were not different between the patients and healthy controls (12). In contrast, in the present study, the TNF-α serum levels were not higher in the patient group. However, serum levels of IL-8 were significantly higher in the patient group.
Angrill et al. performed BAL in 49 patients with stable bronchiectasis and nine control subjects. In this study, BAL levels of TNF- α, IL-6, IL-8 and IL-10 were measured. In comparison with the control group, BAL levels of TNF-α, IL-6 and IL-8 were significantly higher in patients with bronchiectasis. However, IL-10 was not different between patients with bronchiectasis and control subjects. In the present study, patients with bronchiectasis exhibited a significant increase in the BAL levels of IL-8 and a significant decrease in the BAL levels of IL-10 (13).
IL-10 is an anti-inflammatory cytokine that inhibits the effect of pro-inflammatory cytokines such as IL-8 and TNF-α. When the IL-10 level is low, as its inhibitory effect disappears, the levels of IL-8 and TNF-α are expected to be high. Cytokine IL-10 prevents the cytokine synthesis and secretion from T lymphocytes and macrophages. IL-10 and the cytokines that are synthesized from other Th2 cells increase in chronic graft-versus-host disease. From this perspective, IL-10 can be used for the treatment of situations requiring the inhibition of cellular immunity. IL-10 concentrations decrease in induced sputum secretions in patients with COPD and bronchiectasis. These decreased levels of IL-10 can increase lung inflammation (14). Today IL-10 has taken its place in clinical studies that are being conducted on its usability in the treatment of other chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, and psoriasis) in which steroid resistant patients were included.
The levels of IL-10 in serum and BAL fluid were significantly different between groups. Furthermore, as IL-10 inhibits TNF-α and IL-8 secretions from macrophages, in the current study, IL-10 levels decreased and as it decreased levels of IL-10 cannot inhibit IL-8 and TNF-α secretions, IL-8 and TNF-α levels in serum and BAL fluid increased. However, while the IL-8 levels were significantly higher in the patient group according to the control group, TNF-α levels were not high in the patient group when compared to the control group. If the IL-10 levels increased, the anti-inflammatory process would increase more and the inflammatory process would be limited before the inflammation destructs the bronchus. From this perspective, if a selective activator of IL-10 receptors can be found, the therapeutic potential of IL-10 in bronchiectasis will further increase.
It was shown that IL-6 and TNF-α gene polymorphisms are associated with the development, progression, and severity of some diseases such as arthritis, chronic inflammatory diseases, and diabetes (15-17). In the current study, no statistically significant difference was found in the IL-6 -174 G/C polymorphism and the TNF-α -308 G/A and -238 G/A polymorphism.
Polymorphisms of the two promoter regions that belong to IL-8 cytokine gene were examined. In the current study when the IL-8 -161 allele frequencies of the patient and the control groups were examined, no significant difference was found between the two groups in terms of allele frequencies. There was no significant difference between groups in terms of AC and CC genotypes. The CC genotype increased the disease risk by 1.52 fold according to the AC genotype. However, as this risk is not statistically significant, it is assumed that IL-8 -161 C/A polymorphism does not produce a genetic predisposition to bronchiectasis in terms of alleles and genotypes. However, when the -251 allele frequencies were examined, a significant difference was found between two groups in terms of allele frequencies. The T genotype increased the disease risk by 2.38 fold according to the A genotype and this risk was statistically significant. A significant difference was found between the groups in terms of AT and TT genotypes; TA genotype increased the disease risk by 4.19 fold according to the TT genotype and as this risk was statistically significant. It was surmised that the IL-8 -251 A/T polymorphism produces a genetic predisposition to bronchiectasis in terms of the T allele and TA genotype. Being a TA or AA genotype and possessing the A allele is an increased risk factor for bronchiectasis whereas possessing TT genotype and T allele is a protective factor.
When the literature is examined, most of the studies about IL-8 cover respiratory diseases such as asthma. In a study in which Campa et al. investigated the relationship between lung cancer and -251 A/T polymorphism, it was shown that the levels of IL-8 protein in respiratory tract epithelial cells of individuals with -251 A/T polymorphism who were diagnosed with lung cancer was higher it was associated with smoking addiction (18). In another study conducted by Arınır et al., a correlation between IL-8 -251 A/T polymorphism and chronic obstructive lung disease was seen. A positive correlation with the severity of asthma was found in individuals with this polymorphism (19). Additionally, the current study showed that in respiratory tract diseases that are associated with inflammation, the IL-8 -251 A/T polymorphism may be an increased risk factor in the pathogenesis of the disease. In this case, the presence of IL-8 -251 A/T polymorphism shows it may produce a genetic predisposition to disease. However, to claim this, it is necessary to perform this study in larger scaled groups by increasing the number of patients.
In the current study when the IL-10 -592 allele frequencies in patient and control groups were examined, there was no significant difference between the two groups in terms of allele frequencies. No significant difference was detected between the groups in terms of AA and CC genotypes; however, a statistically significant difference was found between AC genotype numbers, and AC genotype frequency was significantly higher in the study group compared to the control group. AC genotype increased the disease risk by 5.71 fold according to the CC genotype and this risk was statistically significant, thus, it was surmised that the IL-10 -592 C/A polymorphism produces a genetic predisposition to bronchiectasis. When the IL-10 -819 allele frequencies were examined, no significant difference was detected between the two groups in terms of allele frequencies and there was no significant difference between the groups in terms of -819 TT and CC genotypes. However, a statistically significant difference was detected between the groups in terms of TC genotype frequency and TC genotype frequency was higher in the study group compared to the control group. The TC genotype increased the disease risk by 5.06 fold according to the CC genotype and as this risk was statistically significant, thus it was surmised that the IL-10 -819 T/C polymorphism produces a genetic predisposition to bronchiectasis. On the other hand, when the -1082 allele frequencies were examined, there was no significant difference between the two groups in terms of allele frequencies. As no significant difference was found between the groups in terms of GG, AG, and AA genotypes, it was surmised that the IL-10 -1082 G/A polymorphism does not produce a genetic predisposition to bronchiectasis in terms of alleles and genotypes. IL-10 levels are affected by the polymorphism in the promoter region of the gene. Polymorphism is seen at points 1082, 819, and 592 nucleotide reversed from the beginning site of transcription. The IL-1082 A allele caused a decrease in IL-10 level and caused the inflammation to progress in a more severe form (20,21).
One of the limitations of the present study was the predominance of the male gender. Therefore, the researchers were unable to generalize the current results to the female gender. Another limitation of the study was the small number of patients due to the single-center study design. Further studies are warranted with a larger number of patients.
In conclusion, when examining the inflammatory and anti-inflammatory cytokines in bronchiectasis with chronic infection and inflammation, it was revealed that IL-8, an inflammatory cytokine, is high in both serum and BAL and IL-10, an anti-inflammatory cytokine is low in serum and BAL. The treatment method of blocking pro-inflammatory cytokines is used in the treatment of various diseases (such as inflammatory bowel disease, psoriasis, and ankylosing spondylitis). Also in bronchiectasis, the inflammation can be limited just before the inflammation destructs the bronchus by blocking the tissue destruction effect of inflammatory cytokines. Furthermore, the process can be terminated against the inflammation without causing injury, by the activation of the receptors in which IL-10 is effective. The detection of gene polymorphisms related to cytokines in genetic analysis may provide the development of gene therapies in the future for individuals who are thought to have a genetic predisposition.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58. [PubMed]
- Barker AF, Ahmed SY. Bronchiectasis. In: Fishman AP, Elias JA, Fishman JA, et al. eds. Fishman’s Pulmonary Diseases and Disorders. 4th ed. New York, McGraw-Hill, 2008:2183-92.
- Martínez García MA. Bronchiectasis: still an orphan disease? Arch Bronconeumol 2005;41:407-9. [PubMed]
- Bidwell J, Keen L, Gallagher G, et al. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun 1999;1:3-19. [PubMed]
- Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol 1975;94:441-8. [PubMed]
- Olveira G, Olveira C, Gaspar I, et al. Fat-free mass depletion and inflammation in patients with bronchiectasis. J Acad Nutr Diet 2012;112:1999-2006. [PubMed]
- Beeh KM, Beier J, Kornmann O, et al. Long-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPD. Chest 2003;123:778-83. [PubMed]
- Guran T, Ersu R, Karadag B, et al. Association between inflammatory markers in induced sputum and clinical characteristics in children with non-cystic fibrosis bronchiectasis. Pediatr Pulmonol 2007;42:362-9. [PubMed]
- Takabayashi T, Vannier E, Clark BD, et al. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J Immunol 1996;156:3455-60. [PubMed]
- Toews GB. Cytokines and the lung. Eur Respir J Suppl 2001;34:3s-17s. [PubMed]
- Bergin DA, Hurley K, Mehta A, et al. Airway inflammatory markers in individuals with cystic fibrosis and non-cystic fibrosis bronchiectasis. J Inflamm Res 2013;6:1-11. [PubMed]
- Martínez-García MA, Perpiñá-Tordera M, Román-Sánchez P, et al. The association between bronchiectasis, systemic inflammation, and tumor necrosis factor alpha. Arch Bronconeumol 2008;44:8-14. [PubMed]
- Angrill J, Agustí C, De Celis R, et al. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am J Respir Crit Care Med 2001;164:1628-32. [PubMed]
- Takanashi S, Hasegawa Y, Kanehira Y, et al. Interleukin-10 level in sputum is reduced in bronchial asthma, COPD and in smokers. Eur Respir J 1999;14:309-14. [PubMed]
- Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 1998;102:1369-76. [PubMed]
- Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol 1997;34:391-9. [PubMed]
- Cuenca J, Pérez CA, Aguirre AJ, et al. Genetic polymorphism at position-308 in the promoter region of the tumor necrosis factor (TNF): implications of its allelic distribution on susceptibility or resistance to diseases in the Chilean population. Biol Res 2001;34:237-41. [PubMed]
- Campa D, Hung RJ, Mates D, et al. Lack of association between -251 T>A polymorphism of IL8 and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2005;14:2457-8. [PubMed]
- Arinir U, Klein W, Rohde G, et al. Polymorphisms in the interleukin-8 gene in patients with chronic obstructive pulmonary disease. Electrophoresis 2005;26:2888-91. [PubMed]
- Turner DM, Williams DM, Sankaran D, et al. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet 1997;24:1-8. [PubMed]
- Ma SL, Tang NL, Lam LC, et al. The association between promoter polymorphism of the interleukin-10 gene and Alzheimer’s disease. Neurobiol Aging 2005;26:1005-10. [PubMed]


