Bronchiectasis after bronchial thermoplasty
Introduction
Bronchial thermoplasty (BT) is a novel therapeutic option for patients with severe asthma refractory to pharmacotherapy (1,2). The procedure targets airway smooth muscle and relieves the bronchoconstriction characteristic of asthma. Radiofrequency energy provides thermal treatment (65 °C) to the visible (>3 mm in diameter) airways. Treatment is administered using a specialized catheter, introduced via a flexible bronchoscope, in three sessions carried out at 3-week intervals (3). Three international randomized clinical trials (2,4,5) in patients with mild to severe asthma demonstrated the safety of BT, significant long-term improvements in the rate of asthma exacerbation, and an acceptable rate of post-treatment adverse events. Recently, the results of a 5-year follow-up were published, in which there was persistent asthma quality of life questionnaire (AQLQ) improvement and reductions in exacerbation, hospitalization, and emergency room visits. In addition, two systemic reviews confirmed the long-term benefits of BT in patients with moderate to severe asthma (6,7). We believe this is the first report to combine optical coherence tomography (OCT) and a 3-year follow-up with chest CT to evaluate a patient with severe persistent asthma who developed bronchiectasis after BT.
Case presentation
A 49-year-old Chinese male began to wheeze in December 2012, especially after physical activity or coming into contact with irritating smells or cold air. His lung function indicated that the provocative test was positive and he was diagnosed with asthma. When he was treated with Symbicort (160/4.5 µg q12h), his symptoms were first relieved but then recurred. He had a history of well-controlled type II diabetes However, his asthma worsened, and he suffered wheezing, dyspnea, and dripping sweat in March 2013. He was treated with Symbicort (320/9 µg q12h), Montelukast, and Theophylline, and his lung function test indicated mild obstructive ventilatory dysfunction in January 2013 (FEV1% Pred: 75.4%, FVC% Pred: 80.4%, FEV1/FVC 76.4%). Because of his recurrent wheezing even under the above treatment regimen, he was diagnosed severe asthma in 2014. His chest computed tomography (CT) scan performed in June 2014 (Figure 1A,B) was normal. He underwent BT in a tertiary referral hospital in June 2014. During this procedure, the airway was treated with thermal energy three times via bronchoscopy: (I) in the right lower lobe, there were 77 complete tissue activations on June 11, 2014; (II) in the left lower lobe, there were 91 complete tissue activations on July 2, 2014; and (III) in the bilateral upper lobes, there were 154 complete tissue activations on July 23, 2014. After BT, the patient took Theophylline, Montelukast, inhaled Budesonide, and Symbicort (320/9 µg, q12h). His self-monitored peak expiratory flow (PEF) was consistently 650 L/min. However, he complained of intermittent thoracalgia on August 14, 2014, and he expectorated yellow viscous sputum. Then, 21 days after the third BT session, he sustained asthma exacerbation with an ACT score of 20. On September 10, 2014, he was seen at a local hospital affiliated with a medical university, whose WBC 7.15×109/L, neutrophils (Neu)% 60.5% and eosinophils (Eos)% 19.3%. A chest X-ray showed bronchitis and right pleural adhesions. Despite 6 days of oral Cefonicid and IV Aminophylline, his thoracalgia and wheezing continued to worsen. On September 17, 2014, he returned to the tertiary referral hospital where he had undergone BT. His complete blood count was WBC 5.44×109/L, Neu% 57.6%, and Eos% 1.1%. The serum total IgE was 238 KU/L. The neutrophils and eosinophils of induced sputum were 25.5% and 73.5%, respectively. His FeNO was 87 ppb. His lung function test showed a decrease in his FEV1 value to 26.5%. A chest CT scan showed mild bronchiectasis accompanied by inflammation (Figure 1C). After he was treated with Methylprednisolone (40 mg qd) intravenously, oral Theophylline (0.2 q12h) and oral Cefoperazone-sulbactam (2 g q12h for 7 days), his symptoms were relieved.
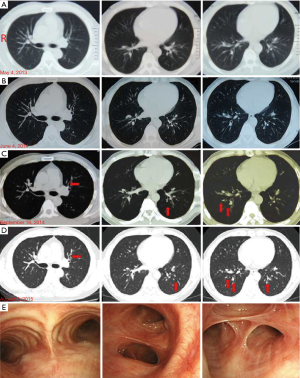
He had recurrent cough and wheezing from October 2014 to August 2015. In August 2015, he was admitted to our hospital. His physical exam was normal. His complete blood count was WBC 8.56×109/L, Neu% 73.9% and Eos% 0.2%. His hepatic function and procalcitonin levels (<0.05 ng/mL) were normal. His serum total IgE was 416 KU/L. The specific IgE for Aspergillus fumigatus was positive. MPO-ANCA and PR3-ANCA were negative. Sputum smear tests for fungi and tuberculosis (TB) were performed in quadruplicate and negative. The induced sputum showed Neu% 87.5% and Eos% 0%. His bronchoalveolar lavage fluid indicated macrophages (Mac)% 96, Neu% 0.5 and Eos% 0. The lung function test results were mild obstructive ventilatory dysfunction (FEV1% Pred: 91.5%, FVC% Pred: 111.4%, FEV1/FVC 66.57%). The paranasal sinus CT scan indicated inflammation in the ethmoid and maxillary sinuses. His chest CT scan showed mild bronchiectasis bilaterally (Figure 1D). Bronchoscopy showed that the right principal bronchus and each segmental bronchus were unobstructed. Airway mucosa was intact. There was a small amount of a white secretion but no neoplasm in the principal bronchus or in the segmental bronchi. The anterior and posterior basal segments of the right lower lobe had a narrow opening surrounded by congestion and a swollen mucous membrane with yellow secretions. The left principal bronchus was also unobstructed. In the posterior basal segment of the left lower lobe, a limited expansion was accompanied by congestion, a swollen mucous membrane, and yellow secretion (Figure 1E). The patient’s symptoms were relieved after 2 days of treatment with oral Prednisone (15 mg qd), Symbicort (320/9.0 µg q12h), Acetylcysteine, Nysfungin tablets, and Acarbose. After discharge from the hospital, he was treated for 16 days with Clarithromycin, oral Prednisone (10 mg qd), Symbicort (320/9.0 µg q12h), isopropyl bromide, and Budesonide aerosol.
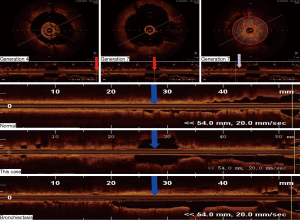
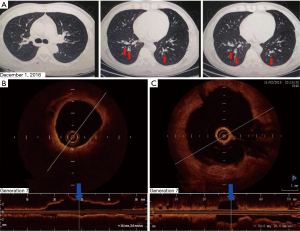
The patient returned to our hospital for reexamination on November 2, 2015. OCT of the bronchi demonstrated that, compared with the 4th-generation bronchus, the 7th-generation bronchus, evaluated in cross-section in the posterior basal segmental bronchus of the left lower lobe, was significantly dilated and filled with sputum bolt (Figure 2). A chest CT scan on December 1, 2016 identified mild bronchiectasis (Figure 3A). OCT performed on February 24, 2017 indicated that the cross-sections of the 6th- to 7th-generation bronchi in the posterior basal segment of the left lower lobe were significantly wider than those of the 4th- and 5th generation bronchi. The distal bronchus was narrow and obstructed but without sputum bolt (Figure 3B,C). From March 2017 to June 2018 the patient coughed up yellow phlegm once every month and he was treated with antibiotics.
Discussion
This patient was a severe asthmatic with blood eosinophilia (19.3%), positive specific IgE for Aspergillus fumigatus, elevated serum total IgE (416 KU/L), negative MPO-ANCA and PR3-ANCA, sputum eosinophilia (73.5%) and nasosinusitis. The chest CT scan and OCT indicated mild bronchiectasis. Because maximal serum total IgE was less than 1,000 KU/L, the patient did not meet diagnostic criteria for allergic bronchopulmonary aspergillosis (ABPA) (7). Although there was blood eosinophilia (>12%) and nasosinusitis, his chest CT scan only indicated mild bronchiectasis, and lung biopsy did not show extravascular eosinophilic infiltrates without symptom about nervous lesion, which did not meet diagnostic criteria for eosinophilic granulomatosis with polyangiitis (EGPA) (8).
In July 28, 2015, the patient’s sputum was cultured for fungi and bacteria and found positive for Streptococcus viridans and Neisseria, but sputum smear tests for fungi and TB performed in quadruplicate were all negative. The patient had no hypoimmunoglobulinemia and no history of using immunosuppressants. The patient also had no congenital disease such as A1AT or cilium defect, as indicated by his normal chest CT before BT (Figure 1A,B). There was also no airway obstruction (Figure 1A,B) and no history of inhalation of toxic substances. This leaves necrosis of smooth muscle after BT as a possible cause of his bronchiectasis. However, the exact pathways that lead to loss of airway smooth muscle—various apoptotic responses, autophagy, necrotic cell death, and other responses—are unclear (9). The number of tissue activation processes performed during the three BT procedures were all significantly higher than the 60 recommended by the current guidelines (10), which may be another cause of his bronchiectasis.
Although CT has been used to detect peripheral airway abnormalities indirectly, it cannot indicate the precise location of airway anatomic changes (11). Chest CT quantification could only accurately evaluate bronchial structure to generation 5–6, for which the caliber of medium-sized bronchi served as the surrogate of small-airway caliber (12). Many studies have indicated that OCT is a useful way to evaluate morphological changes in different portions of airways of chronic obstructive pulmonary disease and asthma before and after BT (13,14). In our hospital, we have evaluated over 10 patients with bronchiectasis by OCT and clearly established morphological changes in different portions of airways of bronchiectasis. Therefore, to make up deficiency of CT, we used OCT to evaluate morphological changes of this patient in our study. According to Figure 2, OCT of the bronchi demonstrated that, compared with the 4th-generation bronchus, the 7th-generation bronchus, evaluated in cross-section in the posterior basal segmental bronchus of the left lower lobe, was significantly dilated and filled with sputum bolt.
Conclusions
While BT is a well-established and safe treatment option for patients who suffer from severe asthma, the causes of bronchiectasis after BT are largely unknown. Ours is the first report to combine OCT and a 3-year follow-up with chest CT to evaluate a patient with severe persistent asthma who developed bronchiectasis after BT. Further studies are needed to fully understand the mechanism underlying BT and its relationship to bronchiectasis.
Acknowledgements
Funding: This research was supported by the Guangdong Science and Technology Project (Nos. 2014A020212368, 2013B021800317) and the Precision Medicine Research of the National Key Research and Development Plan of China (No. 2016YFC0905800).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014;43:343-73. [Crossref] [PubMed]
- Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327-37. [Crossref] [PubMed]
- Xie T, Liu G, Kreuter K, et al. In vivo three-dimensional imaging of normal tissue and tumors in the rabbit pleural cavity using endoscopic swept source optical coherence tomography with thoracoscopic guidance. J Biomed Opt 2009;14. [Crossref] [PubMed]
- Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010;181:116-24. [Crossref] [PubMed]
- Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007;176:1185-91. [Crossref] [PubMed]
- Zhou JP, Feng Y, Wang Q, et al. Long-term efficacy and safety of bronchial thermoplasty in patients with moderate-to-severe persistent asthma: a systemic review and meta-analysis. J Asthma 2016;53:94-100. [Crossref] [PubMed]
- Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013;43:850-73. [Crossref] [PubMed]
- Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990;33:1094-100. [Crossref] [PubMed]
- Janssen LJ. Airway smooth muscle as a target in asthma and the beneficial effects of bronchial thermoplasty. J Allergy (Cairo) 2012;2012. [Crossref] [PubMed]
- Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622-39. [Crossref] [PubMed]
- Bhatt SP, Soler X, Wang X, et al. Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2016;194:178-84. [Crossref] [PubMed]
- Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005;171:142-6. [Crossref] [PubMed]
- Ding M, Chen Y, Guan WJ, et al. Measuring Airway Remodeling in Patients With Different COPD Staging Using Endobronchial Optical Coherence Tomography. Chest 2016;150:1281-90. [Crossref] [PubMed]
- Kirby M, Ohtani K, Lopez Lisbona RM, et al. Bronchial thermoplasty in asthma: 2-year follow-up using optical coherence tomography. Eur Respir J 2015;46:859-62. [Crossref] [PubMed]


