VATS anatomic lung resections—the European experience
Introduction
The advent of thoracoscopic surgery began over a hundred years ago when Dr. Jacobaeus, whilst working as a professor in internal medicine in Sweden, reported his initial experience using a thoracoscope in the diagnosis and treatment of pleural effusions (1). The majority of patients undergoing thoracoscopy at that time had tuberculosis. The development of medical anti-tuberculous medication made the use of thoracoscopy obsolete.
The discovery of fibre-optic light transmission and refinement of instruments led to a rejuvenation of the use of thoracoscopic surgery. In 1978 Miller et al. reported their experience using diagnostic thoracoscopy in previously undiagnosed thoracic disease (2). Alternative diagnostic modalities, available at the time, had failed to provide a diagnosis in every case. In a case series of 11 patients, thoracoscopy facilitated diagnosis in all without morbidity or mortality (2).
Traditional thoracoscope
The original thoracoscope consisted of a hollow tube with a small light bulb over the tip of the scope with a rheostat to control intensity. This resulted in a very limited and often poor view. The only person able to visualise the operative field was the operator. Available instruments were very limited.
Modern scopes
The use of video-assisted imaging systems revolutionised the function of thoracoscopy. In 1952, Fourestier, Gladu, and Valmiere developed a new imaging system which utilised a quartz rod to transmit an intense light beam distally along a telescope. The modern addition of computer chip television cameras further advanced the use of thoracoscopic surgery as it provided a means to project a magnified view of the operative field on to a monitor, freeing both the operating surgeon’s hands, hence facilitating performance of complex procedures.
Further development of 30° and 45° angled viewing scopes has enabled better visualisation of the pleural cavity. Thus far surgeons have had to choose in advance which thoracoscope to use. This restricted their view of the surgical field and intra operative changes of thoracoscope were required to acquire a different viewing angle.
However, a thoracoscope with a variable viewing angle has now been developed. It allows the operator to adjust the viewing angle between 0° and 120° as required during the procedure.
Most authors recommend a 30 degree rigid telescope, a light source and cable, a camera and an image processor in order to perform video-assisted thoracoscopic surgery (VATS) (3,4). Recording facilities and a slave monitor are not essential but an added bonus. Appropriate theatre suites have now been developed. The thoracoscopes used can range in diameter from 3 to 10 mm, depending on the type of procedure being performed. The light source and cable used should also be appropriate for use in VATS. It is recommend that the light source used be an inert gas (e.g., Xenon) mediated “cold light” at 300 W or above. This is higher than that used in other file of minimally invasive surgery as blood in the operating field can absorb up to 50% of the light (5). The use of thinner fibre-optic cables resulted in improved transmission of light.
The invention of thoracoscopic instruments and modification of staplers to allow navigation around pulmonary vessels has led to a rapid increase in VATS procedures. Initially the instruments used in VATS were the same as those used in laparoscopic surgery. However, the increasing use of the VATS approach in thoracic surgery led to the development of tailor made instruments.
The technique
There is no single standardised operative technique in performing a VATS lobectomy. Current popular techniques use a utility incision and 0-3 ports. The original VATS lobectomies were performed using en-masse stapling of hilar structures (6). This approach however, is not recommended in support of individual isolation and ligation of hilar elements, as in open surgery due to oncological principles. VATS anatomical lobectomy for lung cancer was first described in 1992 (3).
Anterior approach
The anterior approach described by Hansen et al. utilises a 3 port technique (7). Both the surgeon and assistant stand on the anterior (abdominal) side of the patient with the surgeon positioned cranially. The approach uses a 10 mm 30° thoracoscope. In contrast to other published anterior approaches the utility incision is made first. It is placed directly over the hilum and the major pulmonary vessels between the breast and the inferior angle of the scapula in the fourth intercostal space anteriorly to the latissimus dorsi muscle (Figure 1).

The approach gives good access to the major vessels in case of major bleeding. Following inspection of the pleural cavity a low anterior 1 cm camera-port is positioned at the level of the top of the diaphragm and anterior to the level of the hilum and the phrenic nerve. Then a further 1.5 cm incision is positioned at the same level but more posterior in a straight line down from the scapula tip and anterior to the latissimus dorsi muscle. The sequence of dissection is the same for all lobes making it an easier technique to teach as both surgeon and assistant are positioned at the same side of the operating table. The first structures to be transected are the major vessels. To prevent air leaks there is minimal handling or dissection of the fissure. This is stapled with the visceral pleura remaining intact as a seal above the lung parenchyma. To facilitate a “no touch technique” the fissure stapling is performed quite late after the majority of the hilar structures have been taken care off.
Most surgeons use an anterior approach. This was popularised by the results published by McKenna Jr et al. (9). The group published a series of 1,100 cases. Their approach utilised 3 sometimes 4 ports. In this series the reported mortality was 0.8% and conversion to a thoracotomy occurred in 2.5% of cases.
Onaitis et al. published a large series on a two port VATS technique (10). Their series reported 500 cases with a surgical conversion rate of 1.6%, an operative and peri operative (30-day) mortality of 0% and 1%, respectively and a median hospital stay of three days.
The first published results on major pulmonary resections performed by a uniportal approach were published by Gonzalez et al. (11).
Posterior approach
The use of the posterior approach in performing a VATS lobectomy was first published by Walker et al. (12). The surgeon is positioned posteriorly to the patient (13). The utility incision is made in the 6th or 7th intercostal space anterior to latissimus dorsi muscle (Figure 2). The camera port is delivered in the auscultatory triangle and the approach utilizes a 0° thoracoscope rather than a 30°. The order of dissection is from posterior to anterior; the oblique fissure is developed first in order to identify and isolate pulmonary arterial branches.
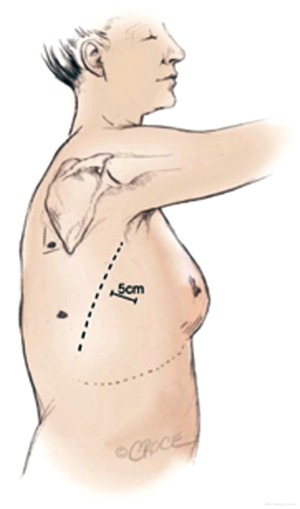
The proposed benefit of the posterior approach is that it provides excellent visualization of the posterior aspet of the hilum facilitating dissection of the bronchi and branches of the pulmonary artery. The sequence of dissection in the posterior approach varies according to the lobe being removed. Furthermore in the posterior approach the tips of the instruments come towards the camera and are therefore easily seen.
Walker et al. published their initial experience having performed 158 cases via this approach (14). Their results showed a combined, inpatient and 30-day outpatient mortality of 1.8% with a conversion to open thoracotomy rate of 11.3%.
The evidence
Mortality and morbidity: open lobectomy
Two recently published large studies suggest that the mortality from open lobectomy is 1-2% with a morbidity of 32-37%.
The ACOSOG Z0030 is a prospective, multi-centre study involving 766 patients who underwent open lobectomy for early-stage non-small cell lung cancer (NSCLC), (T1N0 through T2N1) (15). The authors reported a mortality rate of 1% and an overall complication rate of 37%. However, these results reflect outcomes achieved in expert centres, with carefully selected patients.
Boffa et al. analysed data pertaining patients undergoing lobectomy for NSCLC from the Society of Thoracic Surgeons, database (16). This involved analysis of data on 6,042 patients operated on from 1999 to 2006. The reported mortality rate was 2% and the overall morbidity was 32%. This study included a very heterogeneous patient population and is likely to represent current clinical care and are strikingly similar to the results of the ACOSOG Z0030 trial.
Mortality and morbidity: video-assisted thoracoscopic lobectomy
There are several studies reporting the peri operative outcomes following VATS lobectomy (9,10,17-19). The Cancer and Leukemia Group B 39802 trial was published in 2007 by Swanson et al. (17). This prospective, multicentre study was designed to assess the peri operative outcomes of 127 patients undergoing VATS lobectomy for early NSCLC. Peri operative mortality was 2.7%. Conversely, the peri operative morbidity rate was only 7.4%. However, this was a small group of highly selected patients.
In the largest series published to date McKenna et al. reported a 0.8% mortality rate with a morbidity rate of 15.3% (20). A systematic review conducted by Whitson et al. included 39 studies with 3,256 thoracotomy and 3,114 VATS patients. The authors found that VATS lobectomy was associated with a lower morbidity rate, a shorter chest tube duration and shorter length of hospital stay (21).
Data from several prospective and large retrospective studies also confirm that VATS lobectomy compares favourably with open lobectomy (10,14,20,22-26). The use of VATS reduces morbidity rates to 7.7-24.1% and mortality to 0.8-2.5%. The reported lower morbidity rates included shorter duration of air leak, lower incidence of post operative pneumonia and arrhythmias.
Safety
The initial concerns regarding the intra operative safety of VATS lobectomy have not born fruition. Flores et al. reported only 13 major intra operative complications having operated on 633 patients over 8 years (27).
Another similar series of 410 patients reported only three major intra operative complications requiring emergent conversion (28).
Pain and quality of life
Demmy et al. compared VATS vs. open lobectomy in patients with unfavourable risk factors (29). The authors reported that despite case matching VATS yielded shorter hospital stay (5.3±3.7 versus 12.2±11.1 days, P=0.02), shorter chest tube durations (4.0±2.8 versus 8.3±8.9 days, P=0.06), and earlier return to full preoperative activities (2.2±1.0 versus 3.6±1.0 months, P<0.01). The authors also noted that pain was noticeably better in the VATS group (none or mild, 63% versus 6%; severe, 6% versus 63%; P<0.01) at 3 weeks follow up.
Long et al. conducted a prospective randomised trial comparing quality of life after VATS vs. open lobectomy for clinically early stage NSCLC (30). They found that a month after operation both dyspnoea and pain score were significantly lower in the VATS group (10.9±7.4 vs. 17.4±9.6, P=0.047; 13.7±9.5 vs. 23.0±12.2, P=0.028).
A further prospective, non-randomized study involving 145 patients carried out by Andreetti et al. compared postoperative pain after a VATS lobectomy to a mini-thoracotomy approach (31). They found that the differences in pain scores were significant at 1, 12, 24 and 48 h postoperatively (6.24 vs. 8.74, 5.16 vs. 7.66, 4.19 vs. 6.89 and 2.23 vs. 5.33; P=0.000).
Furthermore, mean forced expiratory volume in 1 second and 6 minutes’ walk test values were better in the VATS group both at 48 h and 1 month following surgery.
Such observations were confirmed by Nagahiro et al. Their results showed faster and improved recovery rates of FVC, FEV1 and vital capacity with VATS lobectomy when compared with open lobectomy (32) at one and two weeks following surgery.
Oncological validity
Lymph node dissection is an essential part of any lung resection for lung cancer. Inadequate lymph node dissection results in inappropriate staging.
Both the National Comprehensive Cancer Network and The European Society of Thoracic Surgeons (ESTS) have developed comprehensive guidelines regarding adequate mediastinal lymphadenectomies (33).
There was though initial scepticism concerning the adequacy of lymph nodal dissection with VATS.
Studies so far though, have demonstrated comparable adequacy and operative mortality and morbidity with lymph node dissection when comparing VATS to open lobectomy (34).
A recent retrospective review of 770 patients with cN0-pN2 non-small lung cancer (VATS =450, open =320) by Watanabe et al. (35) looked at the total number of lymph nodes, nodal stations, mediastinal nodes and stations sampled during systematic lymph node dissection by VATS vs. open lobectomy. They observed no differences in any of these four categories. These findings are further supported by the ACSOG Z0030 trial (n=752, VATS =66, open =686) were a similar number of LN and LN stations were assessed (36) regardless of technique employed.
Competition with systemic/adjuvant therapy
Petersen et al. conducted a study of patients who underwent anatomic resection (37). They specifically looked at whether a thoracoscopic lobectomy was associated with a higher rate of completion of adjuvant chemotherapy when compared to open lobectomy. They reviewed 100 consecutive patients with NSCLC who underwent lobectomy and received adjuvant chemotherapy. They analysed the time to initiation of chemotherapy, percentage of planned regimen received, number of delayed or reduced chemotherapy doses, toxicity grade, length of hospitalization, chest tube duration, 30-day mortality, and major complications. There were 43 patients in the thoracotomy group and 57 in the VATS group. All patients received a complete resection and there were no conversions. Patients who underwent thoracoscopic lobectomy had fewer delayed (18% versus 58%, P<0.001) and reduced (26% versus 49%, P=0.02) chemotherapy doses. A total of 61% of patients who underwent thoracoscopic resection received 75% or more of their planned adjuvant regimen without delayed or reduced doses compared to 40% in the open group (P=0.03).
The immune response
There have been four studies that have looked at the acute-phase reactants and cellular immune responses in patients who received VATS vs. open lobectomies. All four studies show that VATS lobectomy resulted in a lesser degree of inflammatory response (lower interleukin and C-reactive protein levels), reduced postoperative reduction in CD4 and natural killer cells, and reduced impairment of cellular cytotoxicity than open lobectomy (38-42). These results could explain the superiority of VATS lobectomy in morbidity and mortality in comparison with open lobectomy. It remains to be seen whether this difference in biological response translates to a superior long-term outcome.
Cost effectiveness
A study by Swanson et al. compared hospital costs and peri operative outcomes for VATS and open lobectomy procedures in the United States in 3,961 patients (43). Of these 2,907 underwent a lobectomy via open approach and 1,054 via a VATS approach. Hospital costs were higher for open versus VATS at $21,016 and $20,316 respectively (P=0.027).
These findings concur with the findings of Casali et al. They compared the costs of VATS and open lobectomy in 346 (93 VATS lobectomy, 253 thoracotomy) patients operated on between January 2004 and December 2006. The authors reported that the overall cost for a VATS lobectomy was €(8,023±565) compared to the cost of an open lobectomy at €(8,178±167) (P=0.0002). They found that although theatre costs for a VATS lobectomy were higher [€(2,533±230) versus €(1,280±54) for an open lobectomy (P=0.00001)] critical care and LOS were lower in the VATS group resulting in a net saving when performing a VATS lobectomy.
European trends
It is difficult to record the exact number of VATS cases being performed across Europe. However, the ESTS collated data from 235 units across Europe. The database has 56,656 recorded procedures with clinical information on more than 43,330 lung resections. Data analysis demonstrated that the number of VATS procedures dramatically increased from 10.7% between 2007-2009 to 18.8% between 2010-2012. Furthermore the VATS lobectomy rate increased from 2.7% to 11.3% between these two periods.
There is though a large variation in VATS practice across Europe. Several reasons might prevent units from embracing VATS surgery. These include, but are not limited to, overall Centre experience in VATS surgery, cost implications and initial capitol investment in instrumentation, cultural approach and trust to VATS surgery, theatre capacity and cancer target breaches and perceived complexity of the procedure.
Denmark has the highest VATS resection rate across Europe with 55% of lobectomies being performed via a VATS approach across the country in 2011. These cases are split between four specialist units performing between 100 and 325 lung cancer surgeries per annum.
The group in Copenhagen has the largest experience in Europe with more than 1,500 cases performed and 80% of procedures being carried out via VATS. Their experience is evidenced in literature (7,8,13-15).
Despite the fact that the first VATS lobectomy was performed in Italy in October 1991 by Roviaro, the uptake of procedure has been relatively slow in Italy. Between then and December 2012 a total of 1,366 VATS lobectomies have been carried out in Italy. Twelve centers performed over 30 cases and only three centers performed over 100 cases.
In Norway and Sweden several VATS lobectomy programs have started but as yet no one single unit has performed over 100 cases. Similarly Germany, in recent survey of 39 respondents revealed a VATS resection rate of 10%, with all performed via the anterior approach. Two units have performed over 400 cases and seven over 100 cases.
There are 13 units in Austria of which 10 have established VATS lobectomy programs; 3 of these centers have performed over 100 cases (Spring 2013). In the majority of these centers VATS is performed by few surgeons. A total of 1,000 cases have been performed nationally with the VATS resection rate approaching 50% in active centers.
In Switzerland there are 9 public thoracic surgery centers and an unknown number of private centers. Only 2 of the public centers have done over 100 cases as most VATS lobectomy programs started in 2009 or later.
Of the 46 centers surveyed in Spain, 3 did not do VATS lobes, and 22 answered positively. Over 2,000 cases have been performed in total. These procedures were done either via a single port or 3 port approach. The first published results on major pulmonary resections performed by a uniportal approach come from Dr. Boffa et al. from Corunia, Spain (16).
In the Netherlands major centralization of thoracic surgery services and VATS programs began in 2006. One unit has performed over 500 cases and according to the Dutch lung cancer registry VATS lobectomies superseded open in 2012.
The data collated by the Society of Cardiothoracic Surgeons of Great Britain and Ireland shows that VATS resection rate has increased from 2% in 1993 to 14% in 2011. Data from the subsequent years is not yet published.
Conclusions
There is now enough body of evidence to suggest that VATS lobectomies offer a better outcome to cancer patients than open lobectomies. Selection remains the single most important factor to replicate results of studies. We are unlikely to be able to ethically justify a prospective, randomized comparison between open and VATS lobectomy.
This leaves us reliant on the best available current evidence. The current review confirms that VATS lobectomy is a superior procedure associated with lower peri operative morbidity and mortality than open lobectomy. It offers equivalent oncological results, is cost effective, and allows quicker return to social activitie.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Luh SP, Liu HP. Video-assisted thoracic surgery--the past, present status and the future. J Zhejiang Univ Sci B 2006;7:118-28. [PubMed]
- Miller JI, Hatcher CR Jr. Thoracoscopy: a useful tool in the diagnosis of thoracic disease. Ann Thorac Surg 1978;26:68-72. [PubMed]
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Video-assisted thoracic surgery: basic technical concepts and intercostal approach strategies. Ann Thorac Surg 1992;54:800-7. [PubMed]
- Walker WS. Video-assisted thoracic surgery (VATS) lobectomy: the Edinburgh experience. Semin Thorac Cardiovasc Surg 1998;10:291-9. [PubMed]
- Berber E, Siperstein AE. Understanding and optimizing laparoscopic videosystems. Surg Endosc 2001;15:781-7. [PubMed]
- Lewis RJ, Caccavale RJ. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy (VATS(n)SSL). Semin Thorac Cardiovasc Surg 1998;10:332-9. [PubMed]
- Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9. [PubMed]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [PubMed]
- Walker WS, Carnochan FM, Pugh GC. Thoracoscopic pulmonary lobectomy. Early operative experience and preliminary clinical results. J Thorac Cardiovasc Surg 1993;106:1111-7. [PubMed]
- Richards JM, Dunning J, Oparka J, et al. Video-assisted thoracoscopic lobectomy: the Edinburgh posterior approach. Ann Cardiothorac Surg 2012;1:61-9. [PubMed]
- Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [PubMed]
- Swanson SJ, Herndon JE 2nd, D’Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [PubMed]
- Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc 2008;22:298-310. [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60. [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [PubMed]
- Wada H, Nakamura T, Nakamoto K, et al. Thirty-day operative mortality for thoracotomy in lung cancer. J Thorac Cardiovasc Surg 1998;115:70-3. [PubMed]
- Roviaro G, Varoli F, Vergani C, et al. Long-term survival after videothoracoscopic lobectomy for stage I lung cancer. Chest 2004;126:725-32. [PubMed]
- Gonzalez D, de la Torre M, Paradela M, et al. Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 2011;40:e21-8. [PubMed]
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [PubMed]
- Marty-Ané CH, Canaud L, Solovei L, et al. Video-assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg 2013;17:36-43. [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [PubMed]
- Long H, Lin ZC, Lin YB, et al. Quality of life after lobectomy for early stage non-small cell lung cancer--video-assisted thoracoscopic surgery versus minimal incision thoracotomy. Ai Zheng 2007;26:624-8. [PubMed]
- Andreetti C, Menna C, Ibrahim M, et al. Postoperative pain control: videothoracoscopic versus conservative mini-thoracotomic approach. Eur J Cardiothorac Surg 2014. [Epub ahead of print]. [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [PubMed]
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [PubMed]
- Watanabe A, Koyanagi T, Ohsawa H, et al. Systematic node dissection by VATS is not inferior to that through an open thoracotomy: a comparative clinicopathologic retrospective study. Surgery 2005;138:510-7. [PubMed]
- Watanabe A, Mishina T, Ohori S, et al. Is video-assisted thoracoscopic surgery a feasible approach for clinical N0 and postoperatively pathological N2 non-small cell lung cancer? Eur J Cardiothorac Surg 2008;33:812-8. [PubMed]
- Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg 2010;139:976-81; discussion 981-3. [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [PubMed]
- Whitson BA, D’Cunha J, Andrade RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg 2008;86:1735-44. [PubMed]
- Walker WS, Leaver HA. Immunologic and stress responses following video-assisted thoracic surgery and open pulmonary lobectomy in early stage lung cancer. Thorac Surg Clin 2007;17:241-9. [PubMed]
- Craig SR, Leaver HA, Yap PL, et al. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455-63. [PubMed]
- Leaver HA, Craig SR, Yap PL, et al. Lymphocyte responses following open and minimally invasive thoracic surgery. Eur J Clin Invest 2000;30:230-8. [PubMed]
- Yim AP, Wan S, Lee TW, et al. VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 2000;70:243-7. [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [PubMed]


