Early stage lung cancer survival after wedge resection and stereotactic body radiation
Introduction
Lung cancer accounted for 27% of all cancer deaths in the United States of America (USA) in 2014, and is the leading cause of death among cancers in both men and women in the USA (1). Survival however depends largely on stage of diagnosis, and as screening and early cancer-detection become more widely accepted, lung cancer can be diagnosed more frequently at earlier stages with a marked increase in overall survival (OS) rates (1).
Surgical resection has been emphasized as the standard of care and the most effective treatment for early stage non-small cell lung cancer (NSCLC) (2,3). Although more than 80% of lung cancers are classified as NSCLC and are thus candidates for surgery, approximately 25% of these patients do not undergo surgery (4,5). Some of these patients are judged to be inoperable due to medical conditions and comorbidities, while other patients refuse surgery (2). As an alternative, radiation therapy was developed as an additional treatment for NSCLC (2,6,7).
Stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), was developed in order to provide a minimally invasive treatment that improves accuracy, local control and survival rates (8-10). SBRT delivers high radiation doses to immobilized patients under controlled conditions using guided focused beams. This approach has become widely accepted and adapted as an effective alternative treatment for early stage NSCLC (10-15). A meta-analysis comparing various radiotherapeutic approaches for stage I NSCLC reported that SBRT resulted in higher 5-year OS compared to conventional radiotherapy (16).
The comparative effectiveness of SBRT versus surgery in early stage lung cancer is still a topic of debate. A systematic review examining 2-year survival and local control following SABR in NSCLC patients reported equivalent short-medium term survival outcomes to surgery, and put forth SABR as an alternative to surgery (10). Two randomized controlled trials [STARS (17) and ROSEL (18)] attempted to compare SBRT and surgery in the treatment of NSCLC. However, both trials have been closed early due to low recruitment. A pooled analysis of these two trials suggested a better 3 years survival with SBRT in comparison to surgery (19), although a meta-analysis comparing the effectiveness of SBRT and surgical resection in stage I NSCLC concluded that 3-year survival of sublobar resection and SBRT is comparable for stage I NSCLC patients (14). Although surgery is the recommended treatment for early stage NSCLC, those who support SBRT suggest that, once patients’ characteristics such as age, comorbidities, performance and overall health are taken into account, SBRT can be considered a comparable treatment to limited resection.
Recently, wedge resection has become an alternative type of limited surgery performed on small (<1 cm), peripheral nodes, and in patients with comorbidities who cannot tolerate the resection of a larger part of the lung, or even a complete lobe (20). In these special cases, both wedge resection and SBRT can be considered as less invasive and effective treatment approaches for patients who cannot undergo a larger surgical treatment. Published studies comparing wedge resection and SBRT in the treatment of NSCLC are very limited (21,22).
Given the relevance of determining the most beneficial long-term treatment, the primary objective of this meta-analysis was to compare 5-year OS of wedge resection and SBRT in patients with stage I NSCLC.
Methods
Search strategy and selection criteria
Original research articles reporting 5-year OS of patients with stage I lung cancer undergoing SBRT or wedge resection were identified through the National Library of Medicine and National Institutes of Health PubMed database. The following keywords were included in the search strategy: “Stage I”, “lung cancer”, “SBRT”, “surgical resection” and “SABR”. Research published between 1995 and 2017 was included, and the references of selected articles were searched for additional publications. Articles were first screened by title and abstract, and then by full-text if appropriate.
Studies were considered eligible if they met the following inclusion criteria (Figure 1): (I) patients were diagnosed with stage I lung cancer; (II) data on 5-year OS were provided or could be extrapolated from published results; (III) patients were treated with SBRT or SABR, which are equivalent radiation treatment strategies; (IV) the total dose was administered in 5 fractions or less for domestic studies; (V) the majority of patients were treated with a total dose of >40 Gy; (VI) staging was performed with at least a computed tomography (CT) scan; (VII) surgical procedure was a wedge resection, regardless of whether it was performed via open thoracotomy or video-assisted thoracic surgery (VATS); (VIII) data were reported in English. There was no minimum sample size for inclusion. Studies were excluded according to the following reasons: (I) reviews, meta-analyses, editorials, commentaries, SEER re-analyses, conference abstracts; (II) lack of key information for calculation of 5-year survival; (III) patients with lung cancer more advanced than stage I; and (IV) surgical procedure other than wedge resection.
Data extraction
All relevant characteristics were extracted from each article and recorded including author names, year of publication, duration of study, country of study, number of patients treated with SBRT or wedge resection, gender, median or mean age, histology, tumor stage, 5-year survival, median and range of follow-up, the dose/fraction radiation criteria, and if the study was performed on operable or inoperable patients. The primary endpoint of this study was 5-year OS. Data extraction was performed by two independent researchers (SR and WL-C). Data extraction was then performed independently using a standardized data extraction form. Disagreements were resolved by a third reviewer (ET) according to a predefined protocol. The NIH’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to determine the quality of included studies (23).
Data analysis and statistical considerations
Data were processed and analyzed in R (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria), and a summary estimate that accounted for the sample size of each study was calculated. The combined percent survival was calculated using random effect models. Funnel plots were created in order to assess publication bias (Figure S1). Heterogeneity was tested using the Q statistic and the I2 statistic, with I2<25%, 25% to 50% and >50% representing a low, moderate and high degree of heterogeneity, respectively (24,25). Where necessary, median age was converted to mean age (26).
Results
Search results and characteristics of studies
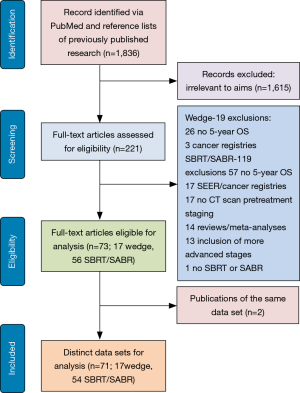
The PubMed search yielded 1,836 potential articles. After screening titles and abstracts, 221 articles were found to be relevant and were reviewed in full text, which resulted in the exclusion of an additional 148 articles. Twenty-nine articles on wedge resection were further excluded due to the absence of 5-year OS data (n=26) and the inclusion of data from cancer registries (n=3). In total, 119 articles on radiotherapy were further excluded due to the lack of 5-year OS data (n=57), stemming from cancer registries and SEER data (n=17), lacking baseline staging with at least CT scan (n=17), reviews and meta-analyses (n=14), including advanced cancer stages (n=13), and reporting on conventional radiation therapy (n=1; Figure 1). This left 73 articles including 71 distinct data sets which were utilized in the present meta-analysis (Tables 1,2, and http://jtd.amegroups.com/public/addition/jtd/supp-jtd.2018.09.140-1.pdf).
Full table

Full table
Wedge resection
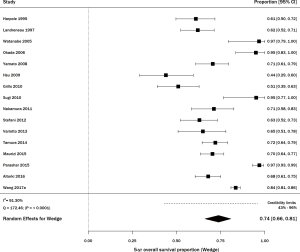
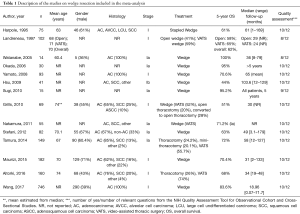
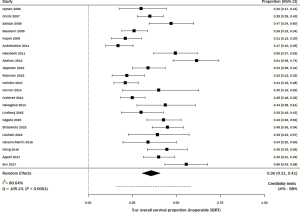
There were 16 studies including 1,984 patients with stage I NSCLC treated with wedge resection for which 5-year OS was available (21,22,27-40) (Figure 2). The sample size of the studies ranged from 14–746 patients. In studies that reported age (n=9) and gender (n=10), the average age was 70 years and the frequency of males was 53%. The range of 5-year OS was 44–100%; the meta-estimate was 74% (95% CI, 66–81%; Figure 2), with significant heterogeneity across studies (Q =172.46, P<0.0001; I2=91.30%). Results from a sensitivity analysis, in which five articles with outlier results were removed, indicated heterogeneity still existed (Q =22.22, P=0.0140; I2=55.00%; Figure S2).
SBRT
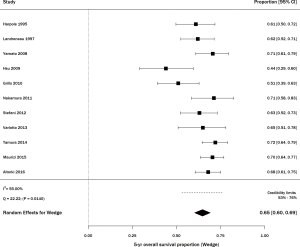
Thirty-six studies including 3,309 patients with stage I NSCLC treated with SBRT/SABR reported 5-year OS (http://jtd.amegroups.com/public/addition/jtd/supp-jtd.2018.09.140-1.pdf). The study size ranged from 20–257 patients. The average age in studies that reported it (n=31) was 75 years, and the average percent of males was 56% (n=32). The range of 5-year OS was 17.0–89.6%, with a meta-estimate of 44% (95% CI, 38–50%; Figure 3). There was significant heterogeneity when pooling results (Q =423.55, P<0.0001; I2=91.74%). Thirteen articles treated the majority of patients with <50 Gy, and among these, the pooled 5-year survival estimate was 48% (95% CI, 36–60%) (15,41-51). The remaining 23 articles treated the majority of patients with ≥50 Gy, and their pooled 5-year survival estimate was 42% (95% CI, 36–48%; data not shown) (11,12,21,52-71).
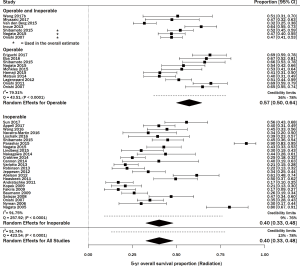
There were 10 studies consisting of 907 patients that reported the 5-year OS of medically operable patients undergoing SBRT (11,12,15,47-50,52,58,65). The meta-survival estimate (Figure 3) was 57% (95% CI, 50–64%), with significant heterogeneity (P<0.0001 and I2=79.31%).
Twenty-five studies consisting of 1,960 patients reported the 5-year OS of medically inoperable patients undergoing SBRT (21,22,41-45,48,49,52-57,59,60,63,64,66,68-71) (Figure 3). The OS was 40% (95% CI, 33–48%) with statistically significant heterogeneity (Q =257.92, P<0.0001; I2=91.75%). Three studies had both operable and inoperable patients (48,49,52), and four articles did not report if the patients were operable or inoperable (46,51,61,62). Results from a sensitivity analysis among inoperable patients receiving SBRT reported high heterogeneity among studies (Q =105.23, P<0.0001; I2=80.04%; Figure S3).
Analysis according to stage
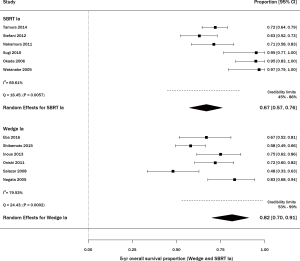
Among studies of wedge resection, there were six that specifically included stage IA, consisting of 345 patients (29,30,32,34,35,39). The percent survival was 83% (95% CI, 65–95%) with statistically significant heterogeneity (Q =24.43, P=0.0002; I2=79.53%; Figure 4). For SBRT, there were 6 articles including stage IA lung cancer, on 228 patients (11,41,46,48,50,53). The percent survival was 67% (95% CI, 57–76%) with statistically significant heterogeneity (Q =16.45, P=0.0057; I2=69.61%; Figure 4).
Direct comparison of wedge resection vs. SBRT
Two articles compared stage I NSCLC patients treated with wedge resection to patients treated with SBRT (Table 2) (21,22). In the first article, 48 patients (50% male) were treated with wedge resection with an average age of 67.5 years, while 137 patients (48% male) were treated with SBRT with a mean age of 73.3 years (21). The comorbidities index was higher for SBRT patients [average score =4.2 (range, 3–10)] than for patients treated with wedge resection [average score =3 (range, 1–6)]. Preoperative lung function was better in patients treated with surgery compared to SBRT [forced expiratory volume in one second (FEV1) of 71% and 49%, diffusing capacity of the lung for carbon monoxide (DCLO) of 56% and 46%, respectively]. Prior to matching, the 5-year OS was higher following wedge resection (~65%) than after SBRT (~21%). After propensity matching (n=17), 5-year OS remained significantly lower (P=0.0003) for patients treated with SBRT (31.7%) compared to wedge resection (86.3%).
The second publication included 123 patients (43.1% male) treated with wedge resection and 97 patients (39.2% male) treated with SBRT (22). Lung function and information on comorbidities were not reported in this article. The 5-year OS was significantly different (P=0.02) between wedge resection (97.7%) and SBRT (89.6%).
Discussion
In the present study, 5-year survival in stage I lung cancer was compared between patients treated with wedge resection or SBRT. This study suggests that wedge resection overall generated significantly higher survival rates than SBRT treatment. It is apparent that studies on SBRT included older patients and patients who are less likely to be operable compared to studies on wedge resection. Patients eligible for SBRT are staged clinically, while surgical patients are staged pathologically, and this may contribute to the difference in outcomes between the two groups. The included publications indicate that SBRT is used in patients who cannot afford surgery because of age or comorbidities, as well as in high-risk and medically inoperable patients; these patients may have worse OS because of their own prognostically negative baseline characteristics. These aspects have to be taken into account when comparing the survival rates between SBRT and wedge resection in retrospective studies.
The present analysis shows that long-term survival for medically operable patients treated with SBRT was significantly higher than the survival observed for medically inoperable patients. From this data, we assessed that patients who would be fit for surgery have a much higher chance of survival when treated with SBRT versus patients who are not candidates for surgery. If a patient who is judged operable refuses to undergo surgery, he/she can be considered for SBRT as an effective, less invasive treatment option.
Another conclusion deriving from this meta-analysis is that the medical status of the patient is very important when assessing survival rates of stage I NSCLC, and when comparing SBRT to surgical resection. There were only 10 studies out of the 36 SBRT studies that reported the 5-year survival of operable patients specifically (11,12,15,47-50,52,58,65), and additional studies on medically operable patients should be conducted to have a larger sample size and provide more definite conclusions. Also very few studies [6 studies for wedge resection (11,41,46,48,50,53) and 6 studies for SBRT (29,30,32,34,35,39)] reported enough information to assess the outcomes of each treatment based on staging. When comparing stage IA patients treated with either wedge or SBRT, improved overall survival was observed with both treatments, although wedge was superior to SBRT. More studies should be conducted on the effectiveness of these treatments in stage IA NSCLC.
To date, only two observational studies directly compared clinical outcomes of SBRT and wedge resection in stage I NSCLC (21,22). The two studies yielded very different conclusions: Parashar et al. reported high survival rates for both procedures (22), while Varlotto et al. indicated very poor 5-year survival rates for SBRT, around 32% in the matched population (21). This result is likely due to the inequal distribution of patient characteristics; patients in the SBRT group had more comorbidities and worse pulmonary function than patients in the wedge resection group. These findings confirm that comorbidities, pulmonary function, prior medical history and performance status are important factors influencing the OS.
The STARS and ROSEL trials were two independent, randomized trails comparing SBRT and surgery in patients with operable stage I NSCLC (17,18). Unfortunately, both trials were closed early due to low accrual. A pooled analysis of these two trials found that patients in the SBRT group had a higher 3-year OS than patients in the surgery group, 95% and 79%, respectively (72). The authors mentioned that lower survival after surgery might be associated with a worsening of comorbidities related to the surgical reduction of lung function. The results of this pooled analysis should be interpreted with caution because only a very small portion of the intended sample size (2.3–3.5%) was enrolled before the trials were closed and follow-up was limited (19).
Limitations
This study has some limitations, the most prominent of which was heterogeneity and the lack of consistency in reporting information in articles spanning over 12 years, from 2005–2017. Study populations were heterogeneous, and their description and characterization were very minimal, which prevented any further in-depth analysis of covariates associated with outcomes. Additionally, many possible confounders such as age, gender, clinical stage, histology, comorbidities, pulmonary function and performance status were missing from many of the studies, which prevented a thorough comparison across procedures. Many studies did not report the location (central or peripheral) of tumors, which influences SBRT regimes and thus survival. Additionally, the biological effective dose (BED) was not always mentioned, which limited the ability to conduct sub-analyses of the survival of patients receiving high doses of radiation (BED >100 Gy). Furthermore, there was variability in the prescribed dose regimes for SBRT studies between studies conducted in the USA, where Medicare defines SBRT as 5 fractions or less, and those conducted internationally. This limits the comparability among SBRT articles.
There was a dearth of studies directly comparing wedge and SBRT, and only two studies included a direct comparison of wedge and SBRT patients (21,22). Another main limitation was that many of the available studies from the initial search limited their follow-up to 2–3 years, and did not provide clear 5-year OS data. Given the excellent curability rates of stage I NSCLC, longer follow-ups are necessary in order to fully evaluate the success of each procedure, and make more definite conclusions about the benefits of wedge and SBRT.
Conclusions
This meta-analysis comparing long-term survival after wedge resection and SBRT in the treatment of stage I NSCLC suggests that wedge resection has higher survival rates. Medically operable patients treated with SBRT have better survival than non-operable patients. More standardized methods for data collection and data reporting are necessary in order to allow comparisons across published studies on wedge and SBRT treatment.
Acknowledgements
We would like to thank Dr. Betsy Becker for her help with the calculation of credibility limits in the forest plots.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors have complied with all ethical standards in publishing.
References
- Torre LA, Siegel RL, Jemal A. In:Ahmad A, Gadgeel S, editors. Lung cancer statistics. Lung Cancer and Personalized Medicine. Springer, 2016;1-19.
- Spiro SG, Porter JC. Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med 2002;166:1166-96. [Crossref] [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [Crossref] [PubMed]
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198-205. [Crossref] [PubMed]
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 2005;128:1461-7. [Crossref] [PubMed]
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung cancer 2003;41:1-11. [Crossref] [PubMed]
- Zimmermann FB, Bamberg M, Molls M, et al. Radiation therapy alone in early stage non-small cell lung cancer. Semin Surg Oncol 2003;21:91-7. [Crossref] [PubMed]
- Videtic GMM. The development of stereotactic body radiotherapy (SBRT) for medically inoperable early stage non-small cell lung cancer: an international phenomenon. J Radiat Oncol 2012;1:3-10. [Crossref]
- Dahele M, Senan S. The Role of Stereotactic Ablative Radiotherapy for Early-Stage and Oligometastatic Non-small Cell Lung Cancer: Evidence for Changing Paradigms. Cancer Res Treat 2011;43:75-82. [Crossref] [PubMed]
- Soldà F, Lodge M, Ashley S, et al. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol 2013;109:1-7. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non–small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685-92. [Crossref] [PubMed]
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [PubMed]
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: a propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol 2010;95:32-40. [Crossref] [PubMed]
- Center MDAC. International Randomized Study to Compare CyberKnife® Stereotactic Radiotherapy With Surgical Resection in Stage I Non-small Cell Lung Cancer. Available online: https://ichgcp.net/clinical-trials-registry/NCT00840749
- Development ZTNOfHRa. A Randomized Clinical Trial of Surgery Versus Radiosurgery (Stereotactic Radiotherapy) in Patients With Stage IA NSCLC Who Are Fit to Undergo Primary Resection. Available online: https://app.dimensions.ai/details/clinical_trial/NCT00687986
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after sublobar resection for early-stage lung cancer: methodological obstacles in comparing the efficacy to lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non–small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Parashar B, Port J, Arora S, et al. Analysis of stereotactic radiation vs. wedge resection vs. wedge resection plus Cesium-131 brachytherapy in early stage lung cancer. Brachytherapy 2015;14:648-54. [Crossref] [PubMed]
- U.S. Department of Health & Human Services. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007;335:914. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Harpole DH, Herndon JE, Wolfe WG, et al. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res 1995;55:51-6. [PubMed]
- Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg 1997;113:691-8; discussion 698-700. [Crossref]
- Watanabe T, Okada A, Imakiire T, et al. Intentional limited resection for small peripheral lung cancer based on intraoperative pathologic exploration. Jpn J Thorac Cardiovasc Surg 2005;53:29-35. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non–small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Yamato Y, Koike T, Yoshiya K, et al. Results of surgical treatment for small (2 cm or under) adenocarcinomas of the lung. Surg Today 2008;38:109-14. [Crossref] [PubMed]
- Sugi K, Kobayashi S, Sudou M, et al. Long-term prognosis of video-assisted limited surgery for early lung cancer. Eur J Cardiothorac Surg 2010;37:456-60. [PubMed]
- Hsu CP, Hsia JY, Chang GC, et al. Surgical–pathologic factors affect long-term outcomes in stage IB (pT2 N0 M0) non–small cell lung cancer: A heterogeneous disease. J Thorac Cardiovasc Surg 2009;138:426-33. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Comparison of the surgical outcomes of thoracoscopic lobectomy, segmentectomy, and wedge resection for clinical stage I non-small cell lung cancer. Thorac Cardiovasc Surg 2011;59:137-41. [Crossref] [PubMed]
- Stefani A, Nesci J, Casali C, et al. Wedge resection versus lobectomy for T1N0 non-small cell lung cancer. Minerva Chir 2012;67:489-98. [PubMed]
- Maurizi G, D’Andrilli A, Ciccone AM, et al. Margin distance does not influence recurrence and survival after wedge resection for lung cancer. Ann Thorac Surg 2015;100; discussion 924-5. [Crossref] [PubMed]
- Altorki NK, Kamel MK, Narula N, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small cT1N0 non–small cell lung cancer. J Thorac Oncol 2016;11:1984-92. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non–small-cell lung cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Tamura M, Matsumoto I, Takata M, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomy versus wedge resection. Indian J Thorac Cardiovasc Surg 2014;30:264-71. [Crossref]
- Wang Y, Wang R, Zheng D, et al. Predicting the recurrence risk factors and clinical outcomes of peripheral pulmonary adenocarcinoma ≤3 cm with wedge resection. J Cancer Res Clin Oncol 2017;143:1043-51. [Crossref] [PubMed]
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. [Crossref] [PubMed]
- Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol 2006;45:787-95. [Crossref] [PubMed]
- Nyman J, Johansson KA, Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer—mature results for medically inoperable patients. Lung Cancer 2006;51:97-103. [Crossref] [PubMed]
- Kopek N, Paludan M, Petersen J, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol 2009;93:402-7. [Crossref] [PubMed]
- Andratschke N, Zimmermann F, Boehm E, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol 2011;101:245-9. [Crossref] [PubMed]
- Inoue T, Katoh N, Onimaru R, et al. Stereotactic body radiotherapy using gated radiotherapy with real-time tumor-tracking for stage I non-small cell lung cancer. Radiat Oncol 2013;8:69. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol 2015;10:960-4. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Eba J, Nakamura K, Mizusawa J, et al. Stereotactic body radiotherapy versus lobectomy for operable clinical stage IA lung adenocarcinoma: comparison of survival outcomes in two clinical trials with propensity score analysis (JCOG1313-A). Jpn J Clin Oncol 2016;46:748-53. [Crossref] [PubMed]
- Miyazaki T, Yamazaki T, Nakamura D, et al. Surgery or stereotactic body radiotherapy for elderly stage I lung cancer? A propensity score matching analysis. Surg Today 2017;47:1476-83. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Salazar OM, Sandhu TS, Lattin PB, et al. Once-weekly, high-dose stereotactic body radiotherapy for lung cancer: 6-year analysis of 60 early-stage, 42 locally advanced, and 7 metastatic lung cancers. Int J Radiat Oncol Biol Phys 2008;72:707-15. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [Crossref] [PubMed]
- Jeppesen SS, Schytte T, Jensen HR, et al. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol 2013;52:1552-8. [Crossref] [PubMed]
- Nakagawa T, Negoro Y, Matsuoka T, et al. Comparison of the outcomes of stereotactic body radiotherapy and surgery in elderly patients with cT1-2N0M0 non-small cell lung cancer. Respir Investig 2014;52:221-6. [Crossref] [PubMed]
- Lindberg K, Nyman J, Riesenfeld Källskog V, et al. Long-term results of a prospective phase II trial of medically inoperable stage I NSCLC treated with SBRT–the Nordic experience. Acta Oncol 2015;54:1096-104. [Crossref] [PubMed]
- Mokhles S, Verstegen N, Maat AP, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer 2015;87:283-9. [Crossref] [PubMed]
- Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non–small-cell lung cancer. J Thorac Oncol 2013;8:192-201. [Crossref] [PubMed]
- Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer 2017;123:3031-9. [Crossref] [PubMed]
- van den Berg LL, Klinkenberg TJ, Groen HJ, et al. Patterns of recurrence and survival after surgery or stereotactic radiotherapy for early stage NSCLC. J Thorac Oncol 2015;10:826-31. [Crossref] [PubMed]
- Wang Z, Li AM, Gao J, et al. Clinical outcomes of CyberKnife stereotactic radiosurgery for elderly patients with presumed primary stage I lung cancer. Transl Lung Cancer Res 2017;6:6. [Crossref] [PubMed]
- Wang P, Zhang D, Guo XG, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Navarro-Martin A, Aso S, Cacicedo J, et al. Phase II trial of SBRT for stage I NSCLC: survival, local control, and lung function at 36 months. J Thorac Oncol 2016;11:1101-11. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer 2017;109:62-7. [Crossref] [PubMed]
- Lischalk JW, Woo SM, Kataria S, et al. Long-term outcomes of stereotactic body radiation therapy (SBRT) with fiducial tracking for inoperable stage I non-small cell lung cancer (NSCLC). J Radiat Oncol 2016;5:379-87. [Crossref] [PubMed]
- Abelson JA, Murphy JD, Trakul N, et al. Metabolic imaging metrics correlate with survival in early stage lung cancer treated with stereotactic ablative radiotherapy. Lung Cancer 2012;78:219-24. [Crossref] [PubMed]
- Appel S, Lawrence YR, Goldstein J, et al. Stereotactic Ablative Body Radiation for Stage I Lung Cancer in Israel: A Retrospective Single-Center Report. Isr Med Assoc J 2017;19:39. [PubMed]
- Cannon NA, Iyengar P, Choy H, et al. Stereotactic ablative body radiation therapy for tumors in the lung in octogenarians: a retrospective single institution study. BMC cancer 2014;14:971. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non–small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non–small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-92. e10.
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]