
Clinical features of Mycoplasma pneumoniae coinfection and need for its testing in influenza pneumonia patients
Introduction
Following the influenza A (H1N1) pandemic in 2009, seasonal influenza remains a critical public health issue (1). Besides neuraminidase inhibitor treatment, influenza pneumonia often requires hospitalization and antibiotic therapy for a possible bacterial coinfection (2). It is difficult to distinguish between influenza pneumonia with and without bacterial coinfection because of the overlapped manifestations (3). Mycoplasma pneumoniae(M. pneumoniae) is a common bacterial pathogen associated with community-acquired pneumonia (CAP) (4,5). Recently, this bacterium has been suggested as a potential cofactor for influenza pneumonia (6). M. pneumoniae is not susceptible to beta-lactam antibiotics recommended by the current guidelines for pediatric CAP (7). Conversely, in young adults with CAP, indiscriminate use of macrolides may lead to the development of macrolide-resistant M. pneumoniae (8). Thus, early detection of M. pneumoniae coinfection in children and young adults with influenza pneumonia could help optimize the antibiotic therapy.
We aimed to investigate the clinical features of M. pneumoniae coinfection in influenza pneumonia patients. Clinical, laboratory and radiographic findings, and outcomes of influenza pneumonia patients with and without the coinfection were compared.
Methods
Study design and setting
This retrospective study was conducted at a tertiary care hospital emergency department (ED) in Seoul, Korea. The ED staffs care for approximately 70,000 adults and 35,000 children annually. We reviewed all patients with influenza pneumonia as a primary diagnosis who had visited the ED, and subsequently underwent M. pneumoniae testing from January 2010 through December 2016. This period comprised 6 whole and 2 partial (2009–2010 and 2016–2017) influenza seasons in Korea.
At our institution, patients with influenza-like illness suggestive of CAP frequently underwent testing for M. pneumoniae and respiratory viruses as part of the initial workup. Further, for microbiological diagnosis, the blood and sputum (if present) of all patients suspected of CAP were analyzed by culture. In some cases, cultures from the pleural fluid or endotracheal aspirates were analyzed. During the study period, the coverage rate of influenza vaccines among Korean children and adults ranged from 45.2% to 49.7%, and from 29.6% to 34.9%, respectively (9). The institutional review board approved this study with a waiver for informed consent (IRB No. S2017-0232-0001).
Study population and definitions
All consecutive influenza pneumonia patients who underwent M. pneumoniae testing were included in the current study. We excluded patients with healthcare-associated pneumonia (confirmed >48 h after ED presentation or <2 weeks after discharge from a hospital), and those with non-mycoplasma bacterial coinfection. Patients with viral coinfection were included due to the little impact on outcome among critically ill patients with influenza A (10).
Influenza pneumonia was defined as laboratory-confirmed influenza plus radiographic pneumonia (peribronchial infiltration, consolidation or pleural effusion) reported by an attending radiologist. M. pneumoniae coinfection was defined as a case having positive results of M. pneumoniae testing including immunoglobulin M (IgM) serology and polymerase chain reaction (PCR).
Influenza and M. pneumoniae testing
For influenza testing, PCR (CFX96, BIORAD, Hercules, CA) or immunofluorescence assay (Sofia Fluorescent Immunoassay Analyzer, Quidel, San Diego, CA, USA) of the nasopharyngeal secretion was performed. The PCR assay was set up to detect the presence of influenza A and B (without subtype information), adenovirus, coronavirus, parainfluenza, rhinovirus, respiratory syncytial virus, human bocavirus, human metapneumovirus, and enterovirus. The sensitivities of the immunofluorescence assay for influenza A and B were 82.2% and 77.9%, respectively (11).
For M. pneumoniae testing, IgM chemiluminescence immunoassay (LIAISON M. pneumoniae IgM, DiaSorin S.p.A., Saluggia, Italy), a qualitative test without a specific titer, was performed using the blood. The turnaround time was less than 3 days. A PCR assay (AmpliSens M. pneumoniae/Chlamydophila pneumoniae-FEP, Ecoli s.r.o., Bratislava, Slovak Republic) was occasionally performed using the nasopharyngeal secretion or sputum.
Data collection
Clinical findings included age (years), gender, ED visits during the influenza seasons, respiratory distress [age-adjusted tachypnea (7), chest retraction, and oxyhemoglobin saturation <90%], temperature, abnormal breathing sounds (crackle and wheezing), and comorbidity (pulmonary, hemato-oncologic, cardiac, renal, neurologic, hepatic, and immunosuppressive diseases) (12). Besides the chronological age, age of 5–15 years was collected as a categorical variable to assess the association of the coinfection and the age group with the highest incidence M. pneumoniae infection (13).
Laboratory findings included white blood cell (WBC), absolute neutrophil, lymphocyte, and platelet counts; concentrations of hemoglobin, creatinine, and C-reactive protein (CRP); the results of influenza and M. pneumoniae testing; and the results of cultures. Radiographic findings included consolidation and pleural effusion on a chest radiograph. By definition of the radiographic pneumonia, all influenza pneumonia patients without the aforementioned radiographic findings had at least peribronchial infiltration.
Outcome variables included the use of neuraminidase inhibitors and macrolides, time-to-neuraminidase inhibitor (time from ED arrival to initiation of neuraminidase inhibitor therapy), time-to-macrolide (time from ED arrival to initiation of macrolide therapy), viral coinfection, severe CAP [defined as per the Infectious Disease Society of America criteria for severe CAP in children and adults (7,14)], hospitalization (overall and at intensive care units), the length of hospital stay (days), and in-hospital mortality.
Statistical analysis
Data are presented as the mean with standard deviation or median with the interquartile range for continuous variables; and as a number and absolute or relative frequency for categorical variables. Student’s t-tests or Mann-Whitney U tests were used to compare continuous variables, and chi-square tests or Fisher’s exact tests were for categorical variables.
Multivariable logistic regression analysis was performed to identify factors associated with M. pneumoniae coinfection. The value of P<0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Windows ver. 21.0 (IBM Corp., Armonk, NY, USA).
Results
Study population
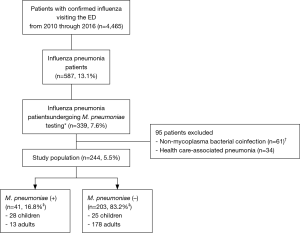
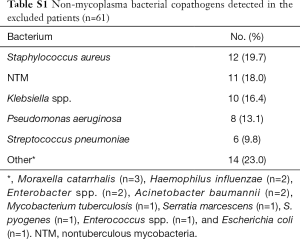
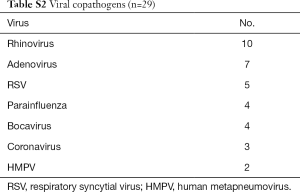
Among a total of 4,465 patients with laboratory-confirmed influenza who visited the ED during the study period, 244 influenza pneumonia patients met all the inclusion criteria (Figure 1). Generally, the influenza pneumonia occurred during the influenza seasons (91.4%), and was due to influenza A (80.7%). The overall rate of bacterial coinfection was 33.4% (102 of the 305 patients). M. pneumoniae coinfection was detected in 41 patients (16.8%; 95% confidence interval, 12.6–22.0%) whose IgMs or PCRs were positive. Of these, 14 patients underwent PCR: 5 were positive for both IgM and PCR, 2 were negative for IgM and positive for PCR, and 7 were positive for IgM and negative for PCR (mutually exclusive). Sixty-one patients with non-mycoplasma bacterial coinfection were excluded (the interested reader can find them in Table S1). Viral coinfections were detected in 29 patients (the interested reader can find them in Table S2).
Full table
Full table
Temporal and age distribution of M. pneumoniae coinfection
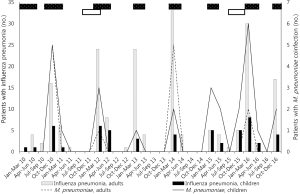
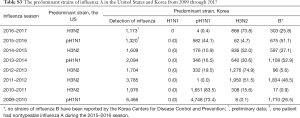
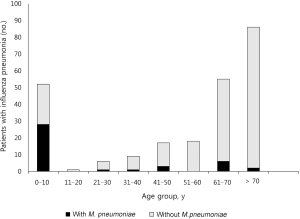
The temporal distribution of influenza pneumonia was consistent with the influenza seasons (Figure 2). The proportion of influenza A in each season generally mirrored the epidemic patterns in Korea (The interested reader can find them in Table S3). Hence, despite the absence of strain information, we speculated that the study population followed the major strain patterns in Korea. M. pneumoniae coinfection was most frequent in the patients aged 10 years or younger (Figure 3).
Full table
Distinction between influenza pneumonia with and without M. pneumoniae coinfection
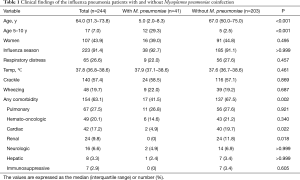
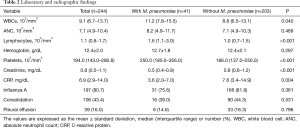
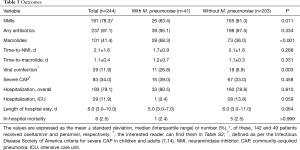
Tables 1 and 2 outline the clinical, laboratory, and radiographic findings, and outcomes. The patients with M. pneumoniae coinfection were younger (usually 10 years or younger), with a higher frequency of age of 5–14 years. This variable was regarded as the age of 5–10 years due to the absence of patients aged 11–14 years in this population (Figure 3). The patients with the coinfection had lower frequency of comorbidity. These patients had higher WBC, lymphocyte, and platelet counts, and lower concentrations of creatinine and CRP, than those without the coinfection. Radiographic findings showed no differences between the two groups.
Full table
Full table
Factors associated with M. pneumoniae coinfection
Multivariable logistic regression analysis showed the age of 5–10 years (adjusted odds ratio, 18.83; 95% confidence interval, 5.899–60.08; P<0.001) as the factor associated with M. pneumoniae coinfection. Lymphocyte count did not reach the statistical significance (for 103 cells/mm3 increment of the count; adjusted odds ratio, 1.001; 95% confidence interval, 1.00–1.001; P<0.001).
Outcomes
Neuraminidase inhibitors were more frequently administered to treat the patients without M. pneumoniae coinfection than those with the coinfection (Table 3). Macrolides were more frequently administered to the patients with the coinfection (68.3% vs. 36.0%, P<0.001). However, this antibiotic therapy showed no association with the better outcome (The interested reader can find them in Table S4). No differences between the two groups were found with respect to the time-to-neuraminidase inhibitor and time-to-macrolide. The patients with the coinfection had more frequent viral coinfections. No other differences for the outcome variables were found between the two groups.
Full table

Full table
Subanalysis performed to the children
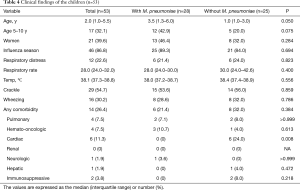
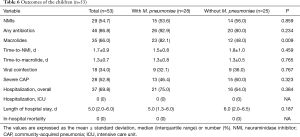
Because 28 of the 41 patients with M. pneumoniae coinfection were children, we performed a subanalysis to assess the association of the age of 5–10 years and the coinfection in the children (n=53) (Tables 4,5). The children with the coinfection tended to belong to the age group more frequently, but did not reach the statistical significance. Due to the small sample size, we could not perform a multivariable analysis. Like the entire study population, macrolides were more frequently administered to the children with the coinfection (82.1% vs. 48.0%, P=0.009) (Table 6). No other differences for the outcome variables were found.
Full table

Full table
Full table
Discussion
We found that M. pneumoniae coinfection in influenza pneumonia patients was associated with the age of 5–10 years, and otherwise clinically indistinguishable from influenza pneumonia without the coinfection in a hospital-based population. The age group is slightly older than the age younger than 2–5 years that is at high risk for influenza pneumonia (12). Thus, M. pneumoniae testing may be useful in early detection of the bacterium in influenza pneumonia patients aged 5–10 years who are at relatively low risk for influenza pneumonia.
Previous reports support the clinical indistinguishability between influenza pneumonia with and without M. pneumoniae coinfection. Clinical assessment of M. pneumoniae pneumonia alone is inaccurate (15). In addition, low WBC count and CRP concentration in influenza pneumonia (16) may hinder distinguishing between influenza pneumonia with and without the coinfection. The radiographic findings of M. pneumoniae pneumonia vary from centrilobular nodule to consolidation (17,18). Despite the unproven impact on the outcome in the current study, the coinfection has a potential for aggravating the disease course. Fischer et al. (19) found a fever of longer duration in M. pneumoniae CAP than in other CAPs.
The implications of M. pneumoniae coinfection in influenza pneumonia should be investigated further because the bacterium is an emerging pathogen in both CAP and influenza pneumonia. A recent population-based study in the United States reported that M. pneumoniae was the most common bacterial pathogen in pediatric CAP (20). Dhanoa et al. (21) reported that M. pneumoniae was the most common bacterial copathogen during the 2009 H1N1 pandemic. In the current study, the bacterium was the most common bacterial copathogen in influenza pneumonia. Conversely, a recent study on the coinfection in M. pneumoniae pneumonia shows that influenza was the most common viral copathogen (22).
M. pneumoniae testing should be performed to optimize the antibiotic therapy in the setting of the coinfection. In this setting, the current guidelines for pediatric CAP, which recommend macrolides as empirical antibiotics only in cases of presumed atypical pneumonia (7), can lead to omission of macrolide therapy. It is hard to clinically presume M. pneumoniae coinfection due to the nonspecific manifestations (15). During influenza seasons, awareness of positive influenza test results could reduce potentially necessary antibiotic therapy (23). This reduction is possibly more harmful to patients aged 5–10 years who have a high incidence of M. pneumoniae infection.
In the current study, macrolides were given more frequently to the patients with M. pneumoniae coinfection without a delay. This finding, along with the clinical indistinguishability, may support the utility of M. pneumoniae testing performed at the ED. This speculation is less likely to be refuted by unproven benefit of macrolide therapy (24) because our topic regarding the coinfection is a matter of the diagnostic plan, rather than a therapeutic modality. The implications of the testing might be less important for adults given the recommendation of macrolides as empirical antibiotics in CAP (14) and lower frequency of M. pneumoniae CAP (25).
The current study has some limitations. First, serology may lead to false negativity, particularly within 1 week of infection or reinfection, or false positivity, such as remote infections or carrier states (26,27). For instance, in the current study, the 7 patients who were positive for IgM and negative for PCR may correspond to false positivities. However, the coinfection rate of 16.8% was less likely to be overestimated. This rate approximates to the rate of PCR-proven M. pneumoniae of 16% that was reported in the 408 patients aged 5–9 years with CAP requiring hospitalization (20). Second, we were unable to know strains of influenza, and yet the strains might affect the clinical features. Third, the coinfection itself does not necessarily mean a secondary bacterial pneumonia because this entity needs the evidence of superinfection (28), and a mere 3–10% of M. pneumoniae infections manifest as CAP (29).
Briefly, M. pneumoniae coinfection in influenza pneumonia may be associated with the age of 5–10 years, and otherwise clinically indistinguishable from influenza pneumonia without the coinfection. Thus, prompt M. pneumoniae testing could contribute to early detection of the coinfection in influenza pneumonia, especially in patients aged 5–10 years who are infrequently contract influenza pneumonia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board approved this study with a waiver for informed consent (IRB No. S2017-0232-0001).
References
- Hui DSC, Lee N, Chan PKS. A clinical approach to the threat of emerging influenza viruses in the Asia-Pacific region. Respirology 2017;22:1300-12. [Crossref] [PubMed]
- Rello J, Pop-Vicas A. Clinical review: primary influenza viral pneumonia. Crit Care 2009;13:235. [Crossref] [PubMed]
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010;38:e91-7. [Crossref] [PubMed]
- File TM. Community-acquired pneumonia. Lancet 2003;362:1991-2001. [Crossref] [PubMed]
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015;386:1097-108. [Crossref] [PubMed]
- Giannattasio A, Brunese L, Ripabelli G, et al. Coinfections with influenza virus and atypical bacteria: implications for severe outcomes? Clin Respir J 2018;12:366-7. [Crossref] [PubMed]
- Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:e25-76. [Crossref] [PubMed]
- Cao B, Zhao CJ, Yin YD, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 2010;51:189-94. [Crossref] [PubMed]
- Korea Centers for Disease Control and Prevention. Sejong, Korea: Ministry of Health and Welfare [cited 2017 Oct 27]. 2010-2015 Korea Health Statistics. Available online: https://knhanes.cdc.go.kr/knhanes/sub04/sub04_03.do?classType=7. Korean.
- Blyth CC, Webb SA, Kok J, et al. The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respir Viruses 2013;7:168-76. [Crossref] [PubMed]
- Lee CK, Cho CH, Woo MK, et al. Evaluation of Sofia fluorescent immunoassay analyzer for influenza A/B virus. J Clin Virol 2012;55:239-43. [Crossref] [PubMed]
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2017 - 2018. Pediatrics 2017;140:e20172550. [Crossref] [PubMed]
- Meyer Sauteur PM, Unger WW, Nadal D, et al. Infection with and carriage of Mycoplasma pneumoniae in children. Front Microbiol 2016;7:329. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Wang K, Gill P, Perera R, et al. Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev 2012;10:CD009175. [PubMed]
- Sohn CH, Ryoo SM, Yoon JY, et al. Comparison of clinical features and outcomes of hospitalized adult patients with novel influenza A (H1N1) pneumonia and other pneumonia. Acad Emerg Med 2013;20:46-53. [Crossref] [PubMed]
- Reittner P, Muller NL, Heyneman L, et al. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol 2000;174:37-41. [Crossref] [PubMed]
- Okada F, Ando Y, Wakisaka M, et al. Chlamydia pneumoniae pneumonia and Mycoplasma pneumoniae pneumonia: comparison of clinical findings and CT findings. J Comput Assist Tomogr 2005;29:626-32. [Crossref] [PubMed]
- Fischer JE, Steiner F, Zucol F, et al. Use of simple heuristics to target macrolide prescription in children with community-acquired pneumonia. Arch Pediatr Adolesc Med 2002;156:1005-8. [Crossref] [PubMed]
- Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015;372:835-45. [Crossref] [PubMed]
- Dhanoa A, Fang NC, Hassan SS, et al. Epidemiology and clinical characteristics of hospitalized patients with pandemic influenza A (H1N1) 2009 infections: the effects of bacterial coinfection. Virol J 2011;8:501. [Crossref] [PubMed]
- Song Q, Xu BP, Shen KL. Effects of bacterial and viral co-infections of mycoplasma pneumoniae pneumonia in children: analysis report from Beijing Children's Hospital between 2010 and 2014. Int J Clin Exp Med 2015;8:15666-74. [PubMed]
- Bonner AB, Monroe KW, Talley LI, et al. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics 2003;112:363-7. [Crossref] [PubMed]
- Chang HY, Chang LY, Shao PL, et al. Comparison of real-time polymerase chain reaction and serological tests for the confirmation of Mycoplasma pneumoniae infection in children with clinical diagnosis of atypical pneumonia. J Microbiol Immunol Infect 2014;47:137-44. [Crossref] [PubMed]
- Foy HM, Kenny GE, Cooney MK, et al. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis 1979;139:681-7. [Crossref] [PubMed]
- Sillis M. The limitations of IgM assays in the serological diagnosis of Mycoplasma pneumoniae infections. J Med Microbiol 1990;33:253-8. [Crossref] [PubMed]
- Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol 2008;8:93. [Crossref] [PubMed]
- Na S, Kim MN, Kim WY, et al. Prevalence and clinical features of pneumonia in patients with laboratory-confirmed pandemic influenza A H1N1 2009 infection in South Korea. Scand J Infect Dis 2011;43:19-26. [Crossref] [PubMed]
- Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 2004;17:697-728. [Crossref] [PubMed]


