The role of induction therapy
Introduction
An estimated 17,990 cases of esophageal cancer are estimated to be diagnosed in the United States annually, with greater than 15,000 deaths attributed to the disease. Over the past 30 years, esophageal cancer 5-year survival rates have improved only slightly and still remain low at 19 percent (1). Although esophageal cancer was traditionally thought of as a surgical disease, the poor cure rates prompted ongoing research into improved treatment modalities. The most promising results have been in induction chemoradiotherapy followed by surgical resection. In this review, we present the current data on induction and adjuvant therapy for locally advanced, resectable esophageal cancer. We illustrate the evolution of practice standards and describe the role of induction chemoradiotherapy in the management of this disease.
Treatment modalities
Surgery alone
In multiple series from the Japanese Oncology Group (JCOG), 5-year survival from monotherapy with surgery alone approached 40% (2,3). Recent Western series have failed to duplicate these results, with 5-year survival rates of 16% (1,4). As such, surgery only is reserved mainly for early stage esophageal cancer (T1-T2) with no nodal involvement (N0) or in urgent circumstances, such as perforation or life-threatening bleeding. More recently, due to concerns over accurate staging of T2 lesions (5), there has been a trend toward treating T2 lesions with induction therapy prior to surgical resection as well.
Radiotherapy alone
Early attempts at single modality therapy resulted in low 5-year survival rates of 4% and 6% percent for surgery alone and radiotherapy alone, respectively (6). Later studies reported slightly improved survival in patients treated with radiotherapy alone. A series from 1985-1994 treated a subset of 101 patients with tumors determined to not be metastatic or locally advanced in patients who were not medically fit for surgery with definitive radiotherapy alone. Staging included endoscopy, barium swallow and occasionally computed tomography (CT). This period was prior to the availability of endoscopic ultrasound (EUS) or positron emission tomography (PET). The majority of patients were given 50 Gray (Gy) of radiation delivered over 15 fractions. The reported 3- and 5-year survival were 27% and 21%, respectively, with a median survival of 15 months (7). Despite these more promising results, treatment for esophageal cancer resulting in better survival rates was sought.
Chemotherapy versus surgery alone
While the standard therapy for thoracic esophageal cancer had been surgical resection, disease-free survival remained poor and adjunct therapies were evaluated. A Japanese phase II study found the combination of cisplatin and 5-fluorouracil (5-FU) offered an improved tumor response above the then standard of cisplatin and vindesine. JCOG 9204 randomized 242 patients with squamous cell carcinoma (SCC) of the thoracic esophagus to receive surgery alone or surgery followed by chemotherapy with cisplatin and 5-FU. The chemotherapy group demonstrated an improved 5-year disease-free survival rate over surgery alone (55% vs. 45%, P=0.037). Overall survival, too, was improved at 61% for dual therapy as compared to 52% for surgery alone (P=0.13) (2).
A recent French study evaluated a similar regimen in 224 patients diagnosed with resectable adenocarcinoma (AC) of the distal esophagus, gastroesophageal junction, or stomach. Patients were randomized to receive induction chemotherapy with cisplatin and 5-FU versus surgical resection alone. The chemotherapy plus surgery group had a disease-free survival of 34%, compared to 19% (P=0.003) in the surgery alone group (8). Five-year survival was 38% versus 24% (P=0.02) in the chemotherapy plus surgery cohort and surgery alone cohort, respectively. Moreover, post-operative morbidity was similar in the two groups.
Induction chemotherapy versus adjuvant chemotherapy
Following the results of JCOG 9204, the same clinical group from Japan randomized over 300 patients with clinical stage II or III SCC of the esophagus in JCOG 9907 (3). Conducted between 2000 and 2006, the cohorts of patients received either preoperative or postoperative cisplatin and 5-FU. The primary endpoint was survival free of disease progression. With a median follow-up of 61 months, the 5-year overall survival in the induction group exceeded the 5-year overall survival in the adjuvant group 55% versus 43% (P=0.04). However, rates of recurrence were equivalent and those in the induction group underwent more surgical procedures for recurrent disease. Of those with recurrence, approximately one third were locoregional in nature. The authors made three conclusions with regards to the improved success of the neoadjuvant administration of chemotherapy: there was more frequent tumor downstaging, R0 resection was more often achieved, and completion of treatment protocols was possible in patients receiving induction chemotherapy.
Induction chemotherapy versus induction chemoradiotherapy
A phase III German study randomized 119 patients with gastroesophageal AC to induction chemotherapy (CT) or chemoradiotherapy (CRT). While the study was closed early due to poor accrual rates, a trend toward improved 3-year survival (47% vs. 27%, P=0.007) was noted in the chemoradiotherapy group. Additionally, pathologic complete response (pCR) was significantly increased (2.0% vs. 15.6%, P=0.03), as was the rate of lymph nodes free of tumor burden (36.7% vs. 64.4%, P=0.01), in patients who received induction chemoradiotherapy (9). This study was criticized, however, for being underpowered.
A similarly underpowered phase II trial from Australia randomized 75 patients with gastroesophageal AC to preoperative chemotherapy or chemoradiotherapy. Akin to the German study the advantages of the chemoradiotherapy with regards to overall survival (32 vs. 29 months, P=0.83) and progression-free survival (26 vs. 14 months, P=0.37) did not reach statistical significance. However, there was significant improvement in pCR (CRT 31% vs. CT 8%, P=0.01) and R1 resection rates (CRT 0% vs. CT 11%, P=0.04) (10).
Chemoradiotherapy
Concurrent chemotherapy and radiation therapy have been used in gastroesophageal cancer as both a definitive treatment and an induction therapy. Radiotherapy is used to treat locoregional tumor growth while chemotherapy is known to both control micrometastatic disease and serve as a sensitization agent for radiotherapy.
Definitive chemoradiotherapy
Definitive chemoradiotherapy is the standard of care for unresectable gastroesophageal tumors and patients medically unfit for surgery as a result of two major trials. RTOG 85-01 demonstrated a 5-year overall survival of 26% versus zero percent in patients with locoregional malignancy. There was, however, a high rate of locally recurrent and persistent disease (11). In an effort to improve the rate of local control, a US Intergroup study (INT 0123) randomized 236 patients all to receive definitive chemoradiotherapy with either 50 Gy (same dose used in RTOG 85-01) or 64 Gy. The increased dose neither yielded improved survival nor locoregional control (12).
Induction chemoradiotherapy
There are five completed randomized trials comparing neoadjuvant CRT to surgery alone (Table 1). Two trials are described in this section in detail. The remaining three trials are included in the meta-analyses reported in the following section. CALGB 9781 was a US study that failed to meet accrual goals of 475 patients but enrolled 56 patients (75% AC, 25% SCC) between 1997 and 2000. The patients were assigned to trimodality therapy (cisplatin and 5-FU concurrent with 50.4 Gy of radiation therapy) or surgery alone with a median follow-up of 6 years. Trimodality demonstrated an improved median survival of 4.5 years compared to 1.8 years in patients undergoing surgery alone (P=0.02). Five-year survival was 39% versus 16% [95% CI of OS hazard ratio (HR) 1.46 to 5.69], in favor of trimodality therapy. Lastly, a pCR rate of 40% was noted (4). However, this study must be considered in the context of incomplete accrual (56 accrued/475 targeted patients) and the relatively low survival with surgery alone.
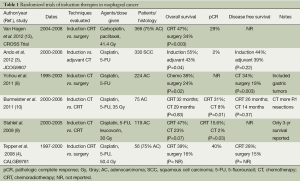
Full table
The Dutch CROSS trial, considered the best evidence regarding induction therapy, enrolled 366 patients (75% AC, 23% SCC) who were randomized to trimodality therapy with carboplatin and paclitaxel plus 41.4 Gy of radiation versus surgery alone for stage II and III esophageal and GE junction tumors. The trimodality group demonstrated a significantly better median overall survival (49.4 vs. 24 months, P=0.003) and higher R0 resection rate (92% vs. 69%, P<0.001). pCR was seen in 29% of patients in the trimodality group. Toxicity was low with chemoradiotherapy (7% with grade 3 hematologic effects) and preoperative treatment did not result in higher postoperative morbidity or early mortality in this group as compared with the surgery alone group. Patients treated with induction chemoradiotherapy followed by surgery had a 34% lower risk of death during follow-up (HR 0.657; 95% CI, 0.495 to 0.871; P=0.003) (13).
Induction chemoradiotherapy meta-analyses
A recent meta-analysis reviewed six randomized studies (n=929) comparing definitive chemoradiotherapy to surgery either alone or with induction therapy. An overwhelming majority of patients included in this meta-analysis had SCC (810/929). Despite variability in the exact therapeutic regimen (total 30-46 Gy of radiation in patients receiving induction therapy; total 45-71 Gy of radiation in patients receiving definitive therapy; infusion versus bolus injection of leucovorin, cisplatin or carboplatin, and paclitaxel), the results were relatively consistent. Overall survival was equivalent between definitive medical therapy and surgery (HR 0.98; 95% CI, 0.8-1.2; P=0.84). There was trend toward higher rate of local recurrence (HR 1.54; 95% CI, 1.2-1.98; P=0.0007) and cancer-related deaths (HR 1.19; 95% CI, 0.98-1.44; P=0.07) in the medical treatment arms. However, treatment related mortality was lower (HR 0.16; 95% CI, 0-0.89; P=0.001) and protocol compliance was better in the nonoperative arms (14).
The most recent meta-analysis on esophageal cancer therapy reviewed 24 trials from 1983-2004 including comparisons of neoadjuvant chemoradiotherapy versus surgery alone (n=1,854), neoadjuvant chemotherapy versus surgery alone (n=1,981), and neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy in patients undergoing resection (n=194). The hazard ratio for all-cause mortality for neoadjuvant chemotherapy (HR 0.87; 95% CI, 0.79-0.96; P=0.005) and neoadjuvant chemoradiotherapy (HR 0.78; 95% CI, 0.70-0.88; P<0.0001) demonstrated a survival benefit of induction therapy (either chemotherapy or chemoradiotherapy) over surgery alone. Chemoradiotherapy maintained its advantage across histologic subtypes (absolute 2-year survival benefit 8.7%), and AC was more sensitive to either treatment than SCC. The advantage of chemoradiotherapy over chemotherapy as a preoperative treatment was apparent (HR 0.88; 95% CI, 0.76-1.01) but not statistically significant (P=0.07). There was no significant increase in post-operative mortality in the neoadjuvant treatment groups (15).
Discussion
The diagnosis of esophageal cancer typically portends a poor prognosis, with 50-60% of patients presenting with incurable locally advanced or metastatic disease. The two main histologic tumors types are SCC and AC. While the incidence of AC is increasing in the United States, worldwide SCC is the predominant tumor type. SCC and AC behave quite dissimilarly and there is little doubt that they represent two different diseases with varying pathogenesis, epidemiology, tumor biology, and outcomes. This difference is reflected in the 2010 TNM staging system, which provides separate stage groupings for SCC and AC of the esophagus. A comprehensive statistical review of the studies on induction chemoradiation for esophageal cancer from 1992-2009 by Bollschweiler et al. demonstrated that the rate of pCR is equivalent between the two histologies. However, AC required higher radiation doses to achieve pCR than did SCC (16). Until studies investigating the benefit of induction therapy randomize patients not only by treatment but also by histology, the current data on induction therapy will be applied to the management of esophageal cancer regardless of histology.
Poor outcomes of surgical treatment alone prompted investigations on the use of adjunct therapies with the goal of improving treatment success. Early studies on radiation therapy demonstrated mild improvement in survival. However, a more notable improvement in overall survival was noted with adjuvant chemotherapy following resection. Later studies comparing induction chemotherapy with adjuvant chemotherapy demonstrated a survival benefit to induction therapy, particularly in that patients were more likely to complete treatment protocols, were more likely to be downstaged, and were more likely to achieve a R0 resection. Efforts towards investigating the role of induction chemoradiotherapy then pursued. While several trials were closed early due to low accrual, several meta-analyses of the multiple existing data sets were possible. It was confirmed that any induction therapy (chemotherapy or chemoradiotherapy) resulted in improved 5-year survival over surgery alone. A trend toward improved survival in patients who had received chemoradiotherapy compared to patients who received induction chemotherapy was noted, but was not found to be statistically significant. The benefit of induction therapy was noted in both SCC and AC, although AC was more responsive than SCC.
Summary
Surgical resection remains the primary treatment for patients with early stage (T1N0) esophageal cancer. In patients with locally advanced disease (≥ T2 or node-positive disease), however, induction therapy plays a critical role towards improving 5-year survival over surgery alone. Patients who received induction chemoradiotherapy appear to have a benefit over those who received induction chemotherapy. However, a clear advantage for chemoradiotherapy has not been established.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [PubMed]
- Zuccaro G Jr, Rice TW, Vargo JJ, et al. Endoscopic ultrasound errors in esophageal cancer. Am J Gastroenterol 2005;100:601-6. [PubMed]
- Earlam R, Cunha-Melo JR. Oesophogeal squamous cell carcinoms: II. A critical view of radiotherapy. Br J Surg 1980;67:457-61. [PubMed]
- Sykes AJ, Burt PA, Slevin NJ, et al. Radical radiotherapy for carcinoma of the oesophagus: an effective alternative to surgery. Radiother Oncol 1998;48:15-21. [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [PubMed]
- Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 2011;47:354-60. [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Pöttgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer--a meta-analysis of the randomized trials. Cancer Treat Rev 2012;38:599-604. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Bollschweiler E, Hölscher AH, Metzger R. Histologic tumor type and the rate of complete response after neoadjuvant therapy for esophageal cancer. Future Oncol 2010;6:25-35. [PubMed]


