Jejunal graft conduits after esophagectomy
Introduction
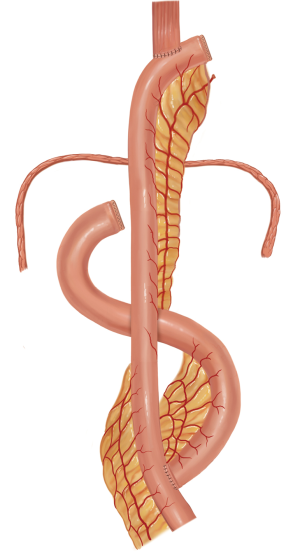
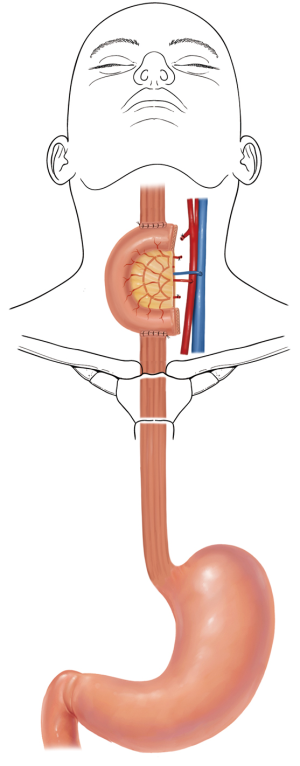
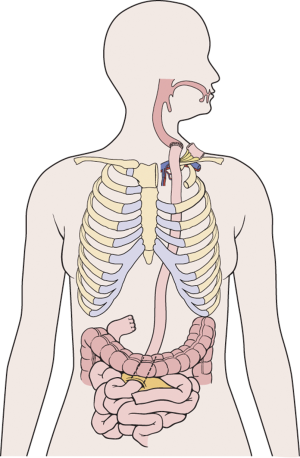
The jejunum is uniquely suitable for esophageal reconstruction because it is relatively abundant, does not require a formal preparation, is typically free of disease, has similar luminal size compared to the esophagus, has intrinsic peristalsis, and may not undergo senescent lengthening to the extent that colon does. The mesenteric vasculature can easily be dissected and mobilized with adequate length to be used as a pedicled or free graft replacing virtually any or all segments of the esophagus. Any region of the esophagus can be replaced by jejunum, whether it is distal esophagus as a Merendino procedure for a vagal-sparing esophagectomy and segmental jejunal reconstruction connected to stomach (Figure 1), mid-thoracic esophagus as a pedicled jejunal interposition or free flap (Figure 2), cervical esophagus as a free segmental interposition (Figure 3), or the entire length as a long-segment super-charged pedicled jejunal interposition (Figure 4). When used, the jejunum is either pedicled, augmented (“super-charged”), a free segment (requiring microvascular anastomosis of artery and vein), or a combination of the above.
History
Decades of surgical evolution are represented by the history of the development of full-length esophageal reconstruction using a pedicled jejunal flap augmented by cervical or thoracic vascular micro-anastomosis (super-charged pedicled jejunum, SPJ) to recreate esophageal continuity after resection. Although Roux was the first surgeon to replace the esophagus with jejunum in 1907 (1), Longmire was the first to describe a long-segment jejunal interposition with microvascular augmentation (2). Androsov used Longmire’s vascular augmentation technique in 11 patients in 1956 (3). The complexity of the operation precluded widespread use in spite of these early reports demonstrating the technical feasibility of the augmented blood supply to the long-segment pedicled jejunal interposition. The utility of small bowel conduit for esophageal reconstruction was confirmed by Allison et al. (4), who in 1957 reported a 3-year follow-up of most patients having normal nutritive intake and work capacity. Ascioti et al. reported the first large series of pedicled jejunal interposition to replace the entire esophagus in cancer patients by using the “super-charging” technique (5), and this series was updated by Blackmon et al. in 2012 (6). This most recent series of 60 patients represents the largest collection of cases of long segment super-charged pedicled jejunal interposition reported to date, however.
Review of the literature
To obtain data to determine the outcomes of jejunal interposition for esophageal replacement, electronic databases were searched, including MEDLINE (Ovid SP), Scopus, EMBASE (Ovid SP), Science Direct’s full-text database, and the Cochrane Library from January 1990 to September 2013. The search strategies were developed using keywords, adjacency searching, and medical subject headings under existing database organizational schemes. Searches were restricted to English-language articles only. Terms used for the search included jejunum, esophageal neoplasms/surgery, esophagus/surgery, esophagectomy, and conduit. The search was limited to humans. British spelling variations were also included. Additionally, PubMed was keyword searched for newly published articles. Two-hundred and forty-six abstracts were reviewed and an article search was performed on selected abstracts. Additional references from article bibliographies were included as appropriate.
Ten articles were excluded because the English version and/or PDF version was not available for review. Five additional articles were excluded because they did not actually include jejunal conduits for esophageal replacement. In articles in which the authors appeared to re-publish data from the same series, the largest series was used and the smaller, earlier series from the same patient population were either excluded or not included in the tabulated results. Nine review articles were also excluded from inclusion into the summary chart. Case reports or series of three patients or less were excluded. Careful review revealed no randomized controlled trials or meta-analyses in adult literature studying esophageal replacement. A similar technique was used to review the literature for colon interpositions to compare outcomes.
Jejunal interposition
Published articles about jejunal interposition to replace the esophagus are listed in Table 1. A total of 14 studies were selected for final analysis and review. One of the major studies was excluded as it reported results on 760 patients but did not specify the choice of conduit used within the body of the paper (17). The route of reconstruction (retrosternal or posterior mediastinal) selected is noted if discussed within the study. Additionally, peri-operative mortality as reported in the paper, anastomotic leak rate, and graft loss frequency are also reported in Table 1. Overall, retrosternal was the most common route utilized by surgeons with a reported 0-10% mortality, 0-36% anastomotic leak rate, and 5-11% graft loss frequency.
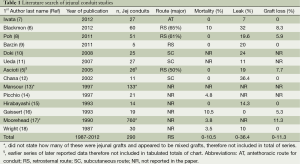
Full table
The route of reconstruction and what the conduit is distally anastomosed to (either jejunum or stomach) may determine the functional outcome. While one strategy may produce more dumping and hypoglycemia, another may result in delayed mixing of food with digestive enzymes and therefore a poorer absorption of nutrients (when connected to jejunum). Pouch reconstruction to create a reservoir for food has shown some promise when the stomach has to be removed as well (19), but this is atypical when a long segment of esophageal replacement is included. Additionally, an intrapleural route, as compared to an extrapleural retrosternal route, may subject the conduit to negative intra-thoracic pressure resulting in both pushing and pulling food in a direction that either enhances digestion or causes aspiration.
Physiology of a jejunal conduit compared to stomach and colon
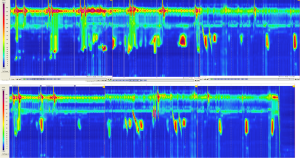
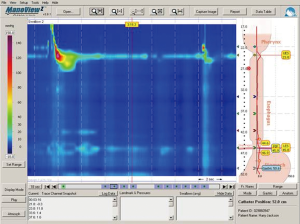
The physiology of a jejunal conduit is unique in comparison to other conduit options of colon and stomach. Manometric evaluation of the jejunal conduit indicates that the jejunum continues to exhibit antegrade segmental contraction as is typical for in situ jejunum (6). This segmental contraction seen with jejunal conduits (Figure 5) does not seem to be coordinated, but appear to assist in evacuation of the conduit. Colon interpositions, on the other hand, have also been used as a part of a prospective evaluation and demonstrate poor to no motility (Figure 6) (20). The ability of the colon to stretch over time leads to redundancy in a negative pressure cavity while the jejunal conduit has lesser propensity to do so when noted in rhetorical studies. Additionally, studies have proposed higher anastomotic leakage rate with colonic interpositions possibly because of the intestinal flora compared to the relative sterile environment of the jejunum (10). For a comparison of colon conduits and review of the literature, please refer to Table 2.
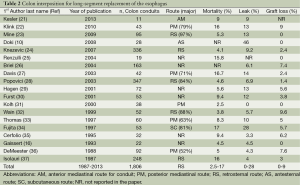
Full table
Post-operative outcomes of jejunal interposition
Postoperative complications are common, including pneumonia, recurrent laryngeal nerve injury, non-occlusive mesenteric ischemia (NOMI), stricture, and graft loss requiring diversion (6,18). Up to 21% of patients suffer from anastomotic stenosis and stricture, as reported by Gaissert et al. (16). Delayed revisional surgery must be performed in many patients for intra-thoracic redundancy resulting in symptomatic partial obstruction, pyloric drainage, and compression of the conduit at the hiatus (6,12,16,18). Peri-operative mortality can be as high as 10.5% (16).
Multivariable analysis for graft loss and leak
Limited number of studies exist that performed logistic regression analysis of those patients who underwent SPJ interpositions and subsequently suffered from conduit loss or leak (6); however, no particular variables have ever been shown to be independent predictors of failure of the SPJ conduit.
Discussion
Patients who have acquired long segment esophageal discontinuity and lack stomach as a viable replacement conduit primarily have two options for reconstruction: jejunum and colon. On the contrary, shorter esophageal segmental replacement has many other options, such as free pedicled forearm skin tubes and folded myocutaneous flaps in addition to the conventional choices. The future may hold many other options, as tissue-engineered 3-dimensional scaffolds repopulated with stem cells have already been used to replace the trachea (38). Esophageal stents have now given us the ability to bridge a disconnected segment of bowel and allow for regrowth of tissue and establish new continuity. Our group has successfully reconnected a distal esophagus to jejunum with a 2 cm separation with the use of stenting alone (39). The addition of antibiotics, stem cells, chemo-attractants, and other materials may enhance healing and re-growth of healthy tissue over the stent matrix. For the purpose of this paper, we focused on those patients where the stomach is not available to create an esophageal conduit, thus rendering the patient to either undergo jejunal or colon interposition to re-establish continuity.
A thorough search of the literature demonstrates the widespread use of jejunum, either as a free, pedicled, or free- and pedicled-graft with acceptable results. Our institutional 10-year experience with SPJ demonstrates the re-establishment or maintenance of GI continuity with acceptable results with a 10% combined 90-day mortality. Closer analysis of the available studies and the circumstances and events that lead to graft loss and leak are multi-factorial and unpredictable. Nonetheless, the technique is replicable and transferable, as evidenced by the successful duplication of an SPJ program at The Houston Methodist Hospital (6). And, although this complex operation can be performed by any thoracic surgeon, the limitations of a jejunal interposition are worth mentioning. Early post-operative course is often associated with significant aspiration and pneumonia secondary to recurrent laryngeal nerve injury which is common with re-operative and/or complex cervical surgery. NOMI is a well-recognized but infrequently encountered complication in under-resuscitated patients who have advancement of tube feeds too early. Additionally, compromise of vascular inflow is highly likely and devastating to the conduit thus requiring frequent monitoring of the indicator flap using dopplers. The management of these patients, close follow up of the grafts (indicator flaps), and nutritional advancement requires a huge inter-disciplinary team of a tertiary care hospital. Therefore, we recommend that such major surgeries be reserved for large volume medical centers where established team of vascular and plastic surgeons as well as nurses, speech therapists, physical therapist, nutritionists, and case managers work together to help the patient recover.
Acknowledgements
Laurissa Gann for her thorough literature search in the different search engines; Elaine Jordan for retrieving manuscripts and journal articles, and Mike de la Flor for his exceptional artwork.
Disclosure: The authors declare no conflict of interest.
References
- Roux C. A new operation for intractable obstruction of the esophagus (L’oesophago-jejuno-gastrosiose, nouvelle operation pour retrecissement infranchissable del’oesophage). Semin Med 1907;27:34-40.
- Longmire WP Jr, Ravitch MM. A new method for constructing an artificial esophagus. Ann Surg 1946;123:819-35.
- Binford RT Jr, Cheraskin E. Clinical problems related to the tongue. Pediatr Clin North Am 1956;919-32. [PubMed]
- Allison PR, Wooler GH, Gunning AJ. Esophagojejunogastrostomy. J Thorac Surg 1957;33:738-48. [PubMed]
- Ascioti AJ, Hofstetter WL, Miller MJ, et al. Long-segment, supercharged, pedicled jejunal flap for total esophageal reconstruction. J Thorac Cardiovasc Surg 2005;130:1391-8. [PubMed]
- Blackmon SH, Correa AM, Skoracki R, et al. Supercharged pedicled jejunal interposition for esophageal replacement: a 10-year experience. Ann Thorac Surg 2012;94:1104-11; discussion 1111-3. [PubMed]
- Iwata N, Koike M, Kamei Y, et al. Antethoracic pedicled jejunum reconstruction with the supercharge technique for esophageal cancer. World J Surg 2012;36:2622-9. [PubMed]
- Poh M, Selber JC, Skoracki R, et al. Technical challenges of total esophageal reconstruction using a supercharged jejunal flap. Ann Surg 2011;253:1122-9. [PubMed]
- Barzin A, Norton JA, Whyte R, et al. Supercharged jejunum flap for total esophageal reconstruction: single-surgeon 3-year experience and outcomes analysis. Plast Reconstr Surg 2011;127:173-80. [PubMed]
- Doki Y, Okada K, Miyata H, et al. Long-term and short-term evaluation of esophageal reconstruction using the colon or the jejunum in esophageal cancer patients after gastrectomy. Dis Esophagus 2008;21:132-8. [PubMed]
- Ueda K, Kajikawa A, Suzuki Y, et al. Blood gas analysis of the jejunum in the supercharge technique: to what degree does circulation improve? Plast Reconstr Surg 2007;119:1745-50. [PubMed]
- Chana JS, Chen HC, Sharma R, et al. Microsurgical reconstruction of the esophagus using supercharged pedicled jejunum flaps: special indications and pitfalls. Plast Reconstr Surg 2002;110:742-8; discussion 749-50. [PubMed]
- Mansour KA, Bryan FC, Carlson GW. Bowel interposition for esophageal replacement: twenty-five-year experience. Ann Thorac Surg 1997;64:752-6. [PubMed]
- Picchio M, Lombardi A, Zolovkins A, et al. Jejunal interposition for peptic stenosis of the esophagus following esophagomyotomy for achalasia. Int Surg 1997;82:198-200. [PubMed]
- Hirabayashi S, Miyata M, Shoji M, et al. Reconstruction of the thoracic esophagus, with extended jejunum used as a substitute, with the aid of microvascular anastomosis. Surgery 1993;113:515-9. [PubMed]
- Gaissert HA, Mathisen DJ, Grillo HC, et al. Short-segment intestinal interposition of the distal esophagus. J Thorac Cardiovasc Surg 1993;106:860-6. [PubMed]
- Moorehead RJ, Wong J. Gangrene in esophageal substitutes after resection and bypass procedures for carcinoma of the esophagus. Hepatogastroenterology 1990;37:364-7. [PubMed]
- Wright C, Cuschieri A. Jejunal interposition for benign esophageal disease. Technical considerations and long-term results. Ann Surg 1987;205:54-60. [PubMed]
- Espat NJ, Karpeh M. Reconstruction following total gastrectomy: a review and summary of the randomized prospective clinical trials. Surg Oncol 1998;7:65-9. [PubMed]
- Dantas RO, Mamede RC. Motility of the transverse colon used for esophageal replacement. J Clin Gastroenterol 2002;34:225-8. [PubMed]
- Kesler KA, Pillai ST, Birdas TJ, et al. “Supercharged” isoperistaltic colon interposition for long-segment esophageal reconstruction. Ann Thorac Surg 2013;95:1162-8; discussion 1168-9. [PubMed]
- Klink CD, Binnebosel M, Schneider M, et al. Operative outcome of colon interposition in the treatment of esophageal cancer: a 20-year experience. Surgery 2010;147:491-6. [PubMed]
- Mine S, Udagawa H, Tsutsumi K, et al. Colon interposition after esophagectomy with extended lymphadenectomy for esophageal cancer. Ann Thorac Surg 2009;88:1647-53. [PubMed]
- Knezević JD, Radovanović NS, Simić AP, et al. Colon interposition in the treatment of esophageal caustic strictures: 40 years of experience. Dis Esophagus 2007;20:530-4. [PubMed]
- Renzulli P, Joeris A, Strobel O, et al. Colon interposition for esophageal replacement: a single-center experience. Langenbecks Arch Surg 2004;389:128-33. [PubMed]
- Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536-41; discussion 541-2. [PubMed]
- Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg 2003;138:303-8. [PubMed]
- Popovici Z. A new philosophy in esophageal reconstruction with colon. Thirty-years experience. Dis Esophagus 2003;16:323-7. [PubMed]
- Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 2001;234:520-30; discussion 530-1. [PubMed]
- Fürst H, Hüttl TP, Löhe F, et al. German experience with colon interposition grafting as an esophageal substitute. Dis Esophagus 2001;14:131-4. [PubMed]
- Kolh P, Honore P, Degauque C, et al. Early stage results after oesophageal resection for malignancy - colon interposition vs. gastric pull-up. Eur J Cardiothorac Surg 2000;18:293-300. [PubMed]
- Wain JC, Wright CD, Kuo EY, et al. Long-segment colon interposition for acquired esophageal disease. Ann Thorac Surg 1999;67:313-7; discussion 7-8. [PubMed]
- Thomas P, Fuentes P, Giudicelli R, et al. Colon interposition for esophageal replacement: current indications and long-term function. Ann Thorac Surg 1997;64:757-64. [PubMed]
- Fujita H, Yamana H, Sueyoshi S, et al. Impact on outcome of additional microvascular anastomosis--supercharge--on colon interposition for esophageal replacement: comparative and multivariate analysis. World J Surg 1997;21:998-1003. [PubMed]
- Cerfolio RJ, Allen MS, Deschamps C, et al. Esophageal replacement by colon interposition. Ann Thorac Surg 1995;59:1382-4. [PubMed]
- DeMeester TR, Johansson KE, Franze I, et al. Indications, surgical technique, and long-term functional results of colon interposition or bypass. Ann Surg 1988;208:460-74. [PubMed]
- Isolauri J, Markkula H, Autio V. Colon interposition in the treatment of carcinoma of the esophagus and gastric cardia. Ann Thorac Surg 1987;43:420-4. [PubMed]
- Gonfiotti A, Jaus MO, Barale D, et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014;383:238-44. [PubMed]
- David EA, Kim MP, Blackmon SH. Esophageal salvage with removable covered self-expanding metal stents in the setting of intrathoracic esophageal leakage. Am J Surg 2011;202:796-801; Am J Surg 2011;202:796-801; discussion 801. [PubMed]