Pediatric lung transplantation: indications and outcomes
Introduction
Lung transplantation (LTx) is a therapeutic option for children and infants with incurable and end-stage diseases of the lungs or pulmonary vascular system. While LTx in this special age group carries unique challenges, there is ample evidence to suggest that outcomes are similar to those in adults. Pediatric LTx offers the potential for prolonging life expectancy and also improving quality-of- life. The purpose of this paper is to review the most common indications for LTx in pediatric patients and to present available outcomes data for children undergoing this procedure.
The era of LTx began over 50 years ago when Hardy and colleagues performed the first transplant in 1963 in a 58 year-old man with bronchial carcinoma (1). Since that time, significant progress in the field has been made in regards to surgical technique, immunosuppressive regimens, recognition and treatment of allograft rejection, and the development of multidisciplinary and collaborative surgical and medical teams to provide optimal long-term care (2-5). Over 43,000 LTx have been performed in adults with the most common indications being COPD, pulmonary fibrosis and cystic fibrosis (CF) (6).
The first pediatric LTx involved a 16 year-old boy with familial pulmonary fibrosis and was performed in 1987 at the University of Toronto (4). Successful LTx has subsequently been performed in children of all ages, including infants, yet the majority of pediatric cases involve children over the age of 11. The most recent registry data of the International Society for Heart and Lung Transplantation (ISHLT) reports that 1,875 lung transplantations have been performed in pediatric patients, most commonly for a diagnosis of CF (7). There is clear evidence that survival after pediatric LTx has improved in recent years, a trend most reflective of improvement in early survival (8). As the total volume of pediatric transplants is far exceeded by those performed in adults, it is not surprising that the total number of centers providing LTx in children and infants is small. In 2011, only 43 centers reported LTx in children with the majority being located in North America and Europe. In addition, most pediatric centers have very low volumes compared to adult programs, with only one center performing more than 10 transplants per year. The total number of children undergoing LTx each year has been slightly greater than 100 from 2006-2011.
There are several important anatomical, physiological, psychosocial and epidemiological factors that are indeed unique to LTx in children and infants (2,4,9,10). First, the size of both pediatric lung donor and recipient may present special surgical challenges with regards to size matching and bronchial and vascular anastomoses. The immune systems of children, and infants in particular, are immature and developing and therefore unlike those of adults. It has been suggested that young children may have less risk of acute and chronic allograft rejection and therefore have more tolerance of transplantation (11). There is also evidence that certain infectious issues, particularly seasonal respiratory tract viruses, are of paramount importance in pediatric LTx (12,13). Nutrition, gastroesophageal reflux disease, and risk of aspiration may all have direct influence on morbidity and survival in children (14). Another important factor in successful LTx in pediatric patients is appropriate parental support to provide for the very complex post-transplant care. Unreliable psychosocial circumstances can in fact be a major obstacle to long term success (15). Adolescents in particular may struggle with adherence to prescribed therapies as the mature and gain more independence. Taken together, these special considerations in pediatric LTx are important factors to consider in evaluating a potential patient for transplant candidacy.
Indications for pediatric lung transplantation (LTx)
CF is the most common indication for LTx in pediatric patients overall and was the primary diagnosis in 1,063 of 1,875 (57%) children in the IHSLT registry (5). Idiopathic pulmonary arterial hypertension (IPAH) is the second most-common indication for LTx, and 164 cases (9%) have been reported. Other less common but important indications for pediatric LTx include: idiopathic pulmonary fibrosis (IPF), surfactant protein deficiencies and other diseases now more uniformly classified as childhood interstitial lung diseases (chILD), congenital heart disease, and re-transplantation.
There is substantial variability in the indication for LTx among sub-groups of pediatric patients divided by age. For instance, in older children and adolescents aged 11-17 years, CF is the indication for LTx in 70% of cases. However CF becomes less predominant in younger aged patients, representing 53% of LTx in children 6-10 years of age and less than 5% in young children under age 5. The most common indication for LTx among children age 1-5 and 6-10 years of age is IPAH. Congenital heart disease, the chILD syndromes including surfactant protein B deficiency (SP-B), and IPAH are the most common indication for infant LTx. The most common indications for pediatric LTx categorized by age group are demonstrated in Table 1.
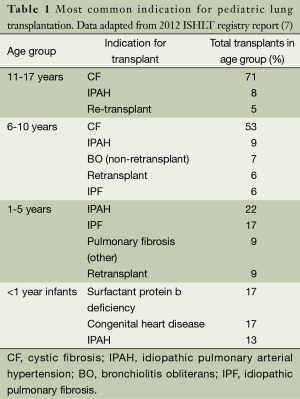
Full table
Cystic fibrosis (CF)
CF is the most common fatal genetic disease affecting Caucasian populations worldwide. This disease is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a chloride channel responsible for ion transport across epithelial cells lining the respiratory tract. Abnormal CFTR results in dehydration of the airways, thick mucus, and poor mucociliary clearance. This causes a cycle of obstruction of the airways by viscous mucus, chronic airway infection, and chronic lung and systemic inflammation. Chronic respiratory failure from CF lung disease is the most common cause of death (16,17). The hallmarks of treatment are airway clearance via chest physiotherapy, aerosolized medications that can help rehydrate and reduce mucus viscosity, anti-inflammatory therapies, aggressive treatment of both chronic and acute on chronic infections, and optimization of key CF comorbidities such as malnutrition and diabetes mellitus (16,18). More recently developed genotype-specific therapies may help correct the underlying CFTR defect which causes CF (19). Survival for CF has improved dramatically over the last several decades with the most recent median survival exceeding 41 years (20). However, despite improvements in treatment and improved survival, LTx remains an important treatment option for advanced CF lung disease in childhood and adolescence. CF is the most common reason for transplant in pediatrics and CF is the third most common indication among adults.
Idiopathic pulmonary arterial hypertension (IPAH)
IPAH is the second most-common indication for LTx in pediatric patients overall, and is the most common indication among children aged 1-5 years. Pulmonary hypertension (PH), in general, is defined by a mean pulmonary artery pressure at rest greater than 25 mm Hg and a pulmonary vascular greater than 3 Woods after three months of age (21). Recent classification strategies by the World Health Organization and the Pediatric Task Force of the 5th World Symposium convening in Nice, France [2013] have further grouped patients with PH into several main categories by main mechanism of elevation in pulmonary artery pressure (22). A detailed description of the classification schema of PH is beyond the scope of this paper, but Group 1 (pulmonary arterial hypertension) diseases which include IPAH, heritable PAH, and PAH associated with congenital heart diseases are the most frequently encountered entities causing end-stage cardiopulmonary disease in pediatric patients.
The natural history of untreated IPAH is one of rapid clinical deterioration and frequent death, often within three years of initial diagnosis. The progression of children with PH may be more rapid than in adult patients (23-25). However, in recent years the development of more effective pulmonary vasodilator medications, in particular the prostacyclin based therapies, has demonstrated clear improvements in survival (21). Despite the benefits of IPAH medications, the ultimate outcome in most pediatric patients is death and therefore LTx remains an important and viable treatment strategy (26). Current guidelines in adults would suggest referral for LTx when patients reach New York Heart Association functional classification of level III to IV, meaning patients who are symptomatic with exertion or at rest. The applicability of these subjective categories in young children may be of limited utility, however there is evidence that children with supra-systemic right heart pressures and those who experienced hemoptysis were at increased risk for death on the waitlist (26). This would suggest that children with IPAH and these poor prognosticating features should be listed early for transplantation.
Interstitial lung disease (ILD) and surfactant protein deficiencies
It has been well-recognized that ILD in pediatric patients differs significantly from that in adults (27). The chILD syndromes have been described as a heterogeneous group of disorders affecting children less than 2 years old with respiratory signs and symptoms (most frequently tachypnea), impairment in gas exchange (hypoxemia) and evidence of diffuse parenchymal lung disease on chest imaging. The American Thoracic Society has recently published clinical guidelines for the diagnosis and management of these patients (28). The chILD syndromes can be sub-divided as those syndromes affecting infants and those not specific to infancy.
The surfactant protein deficiencies are quite rare diseases but are the most common indication among the chILD diseases for LTx in infancy. There have been four surfactant protein deficiency syndromes described including SP-B, surfactant protein C deficiency (SP-C), adenosine triphosphate binding cassette protein member A3 (ABCA3), and thyroid transcription factor (NKX2.1 gene) (29-31). The presentation of the surfactant deficiencies may vary from severe hypoxemic respiratory failure in the newborn period (32) (typical of SP-B) to a more insidious development of tachypnea, hypoxemia and diffuse interstitial changes on chest imaging later in infancy (more typical of SP-C) (33). Diagnosis of these syndromes can be achieved through genetic sequencing technology (28). Perhaps the most important (and most aggressive) surfactant protein deficiency is SP-B, which is recognized as a universally fatal disease and LTx is considered the only viable treatment option (34).
Other important chILD syndromes that may lead to LTx in infants include disorders of lung development such as alveolar capillary dysplasia with misalignment of pulmonary veins (a disease affecting infants in the newborn period that is believed to be uniformly fatal) and growth abnormalities such as neonatal chronic lung disease (bronchopulmonary dysplasia) (35).
BO and re-transplantation
BO refers to obstructive lung disease resulting from bronchiolar inflammation and is described pathologically by circumferential peribronchial fibrosis that can constrict or completely obliterate the lumen of the bronchiole (36). BO can be caused by infectious or non-infectious insults to the airways which trigger the process of inflammation and fibrosis. Post-infectious BO in children is frequently associated with severe viral (adenovirus) or mycoplasma infections (37). Non-infectious BO can occur in children as a consequence of autoimmune diseases, inhalational injuries, and Stevens-Johnson syndrome among others. However, a very important cause of BO is post-transplant in nature. BO can occur as a consequence of pediatric bone marrow transplantation (38,39). Any of these specific etiologies of BO can ultimately manifest in respiratory failure and be an appropriate indication for LTx in children.
The most common group of patients with BO undergoing consideration for LTx is in fact primary lung recipients who develop chronic allograft dysfunction over time. BO remains the major obstacle to long term success in LTx recipients and current treatment options are limited (40). Therefore BO following initial LTx remains an important indication for consideration of re-transplantation. Pediatric patients may be given special consideration for re-transplantation, as achieving an expected graft survival and therefore “good outcome” defined by some standards may not allow a child to reach adulthood. The indications for pediatric lung re-transplant can generally be classified as those patients with chronic allograft dysfunction with BO versus those without BO who suffer graft failure from other causes. A total of 118 pediatric lung re-transplants have been reported, and available data suggests this procedure is most beneficial in patients with chronic graft failure occurring greater than 1 year post-initial transplant (28,41).
Outcomes
Although the most common indications for LTx in pediatric patients differ from those of adults with end-stage lung disease, the available data on outcomes suggest that the success of LTx is quite similar. While survival is certainly the paramount outcome measure for LTx recipients of all ages, other variables such as the incidence of graft rejection, the frequency of key comorbid conditions, the need for re-transplantation, and overall quality of life and functional status are also clinically important.
Survival data
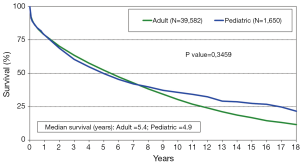
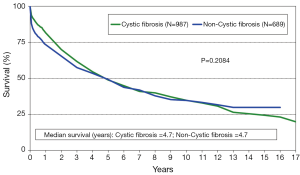
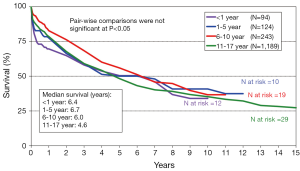
The annual IHSLT Registry report is the most comprehensive database of thoracic LTx performed world-wide (28). Participation in this registry is voluntary but it is believed that this data encompasses the vast majority of pediatric LTx performed each year. In 2011, a total of 43 centers performed LTx in pediatric patients with the vast majority of these centers located in Europe (n=20) and North America (n=18). The 2013 ISHLT registry data of pediatric LTx performed between 1990 and 2011 reports a median survival of 4.9 years for pediatric patients. This observed survival is statistically similar to that of adult LTx recipients (4.9 versus 5.4 years, P=0.3459, Figure 1) Like in adults, there has been a clear improvement in survival when comparing era of transplant, with median survival of 3.3 years among those transplanted between 1988-1999 versus median survival of 5.8 years in those transplanted in the modern era of 2000-2011 (P≤0.001). Pediatric patients with CF have similar survival to those without CF, with median survival of 4.7 years in both groups (Figure 2). While it appears children age 6-10 years may have improved early survival, there is no clear difference in overall long term survival (Figure 3). The 2012 US Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients (OPTN/STRS) data analysis of pediatric LTx performed in the US in 2007-2008 reported post-transplant survival of 96.3% at 30 days, 87% at one year, 60.1% at 3 years, and 49% at 5 years (42).
The most common cause of death in the first 30 days following pediatric LTx is graft failure which accounts for approximately 30% of early mortality (7). Non-CMV infection and graft failure are the most common causes of death from one month to one year post transplant, and account for over 50% of mortality in this time period. Bronchiolitis obliterans syndrome (BOS), like in adults, is the most common cause of death after the first year following pediatric LTx, and represents 40% of deaths at both 1-3 and 3-5 years post-transplant. BOS is responsible for 47% of deaths after 5 years (28). Thus BOS remains the biggest obstacle to long-term survival in both pediatric and adult LTx recipients. This data is consistent with that presented in the OPTN/STRS database (42).
The available data on re-transplants in pediatric patients suggest that outcomes are worse compared to the initial transplant. There were 118 pediatric re-transplants performed between 1994 and 2012, and the approximately 23% of these procedures were performed within 12 months of the original. Three year survival among re-transplants is significantly lower than that of primary transplants (58% vs. 45%, P=0.026.) There was no significant difference between indications for re-transplant when comparing BOS to non-BOS related cases (28). It appears that the best outcomes for pediatric lung re-transplant are achieved in patients that are further than one year removed from initial transplant and are not ventilator dependent (41).
There are multiple studies that highlight survival characteristics and special considerations among specific groups of pediatric LTx recipients. A controversial study regarding the benefit of LTx for children with CF, published by Liou and colleagues, analyzed data from the CF Foundation and United Network for Organ Sharing databases between the years 1992 and 2002 (43). This group concluded that few children with CF achieved an overall survival benefit from LTx. Since that time, a large analysis of adult data from 2005-2009 (the lung allocation score era) demonstrated a strong improvement in adults with CF undergoing LTx (44). Many pediatric LTx centers and leading experts in the field have cited several reasons why the data from Liou at al may not be applicable to individual children with CF in the current era of LTx, citing the transition to the current lung allocation system in the US as well as controversies regarding the statistical analysis and cohort used in the study among others (45,46). There is no clear evidence that the frequency of pediatric LTx for CF has decreased in recent years, although with advances in the care of CF patients it is reasonable to anticipate a future shift towards more transplantations occurring in adulthood as opposed to childhood or adolescence. Over the past decade, it has become clear that CF patients with chronic infection with Burkholderia cenocepacia infection are particularly at risk for poor outcomes following LTx, primarily due to infection in the post-transplant period (47,48). Therefore, infection with B. cenocepacia is considered a contraindication at most centers.
Among diseases other than CF, there is data to suggest equivalent post-transplant survival. For instance, a retrospective single center review of 26 children undergoing LTx for IPAH showed a median survival of 5.8 years and 1- and 5-year survival of 95% and 61% respectively (26). Likewise, a multicenter retrospective chart review of 31 children undergoing LTx for diffuse lung disease (encompassing the chILD syndromes) showed comparable survival compared to children undergoing LTx for other indications (49).
The most common indications for LTx in infants are SP-B deficiency, congenital heart disease, and IPAH. Successful LTx in infants may be particularly challenging due to factors such as donor availability, size of the donor and recipient, risk of post-transplant respiratory viral infection, and other physiological factors such as aspiration risk. Infant LTx is a very rare procedure performed only at a handful of centers. In 2011 only four infant transplants were performed in the US, a number far below the number of heart transplants performed in this age group (42). An analysis of the UNOS database reported similar overall survival among 80 infants (<1 year of age) compared to older children and adolescents (age 1-18 years). This study also suggested an improved conditional survival for those infants surviving at least 1 year (50). This data suggests a potential protective advantage of the immature immune system of infants, and is corroborated by a previous study demonstrating a decreased incidence of allograft rejection among infants (11).
Outcomes other than survival
There appears to be a similar incidence of key post-transplant comorbid conditions following LTx in both pediatric and adult populations. The most commonly encountered co-morbidities at one year following LTx in pediatrics include hypertension, renal dysfunction, hyperlipidemia, and diabetes mellitus. These same conditions increase in frequency in survivors at 5 years post-transplant (7).
It may be challenging to assess functional status and quality of life in pediatric patients who may not be able to express their feelings adequately, and secondary reports from parents or physicians may be confounded by bias. However, the ISHLT registry did report that more than 80% of pediatric LTx recipients were given favorable assessments of functional status as measured by reported Lansky scores (7).
Summary
Pediatric LTx is a viable treatment option for infants and children with end-stage pulmonary diseases. The most common indications for children are CF and IPAH, while the chILD syndromes and congenital heart disease are the predominant indication for infants. Overall survival after LTx in the pediatric population is similar to the expected survival in adults. Chronic allograft rejection remains the biggest obstacle to more prolonged survival, and re-transplantation in select patients may be a reasonable treatment option.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hardy JD, Webb WR, Dalton ML, et al. Lung Homotransplantation in Man. JAMA 1963;186:1065-74. [PubMed]
- Sweet SC. Pediatric lung transplantation. Proc Am Thorac Soc 2009;6:122-7. [PubMed]
- Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med 2011;184:159-71. [PubMed]
- Mendeloff EN. The history of pediatric heart and lung transplantation. Pediatr transplant 2002;6:270-9. [PubMed]
- Huddleston CB, Bloch JB, Sweet SC, et al. Lung transplantation in children. Ann surg 2002;236:270-6. [PubMed]
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Benden C, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official pediatric lung and heart-lung transplantation report--2013; focus theme: age. J Heart Lung Transplant 2013;32:989-97. [PubMed]
- Zafar F, Heinle JS, Schecter MG, et al. Two decades of pediatric lung transplant in the United States: have we improved? J Thorac Cardiovasc Surg 2011;141:828-32, 832.e1.
- Huddleston CB. Surgical complications of lung transplantation in children. Semin Thorac Cardiovasc Surg 1996;8:296-304. [PubMed]
- Huddleston CB. Pediatric lung transplantation. Curr Treat Options Cardiovasc Med 2011;13:68-78. [PubMed]
- Ibrahim JE, Sweet SC, Flippin M, et al. Rejection is reduced in thoracic organ recipients when transplanted in the first year of life. J Heart Lung Transplant 2002;21:311-8. [PubMed]
- Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis 2009;11:304-12. [PubMed]
- Liu M, Mallory GB, Schecter MG, et al. Long-term impact of respiratory viral infection after pediatric lung transplantation. Pediatr Transplant 2010;14:431-6. [PubMed]
- Benden C, Aurora P, Curry J, et al. High prevalence of gastroesophageal reflux in children after lung transplantation. Pediatr Pulmonol 2005;40:68-71. [PubMed]
- Devine KA, Reed-Knight B, Loiselle KA, et al. Predictors of long-term health-related quality of life in adolescent solid organ transplant recipients. J Pediatr Psychol 2011;36:891-901. [PubMed]
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 2006;173:475-82. [PubMed]
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992-2001. [PubMed]
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918-51. [PubMed]
- Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72. [PubMed]
- Cystic Fibrosis Foundation Patient Registry, 2012 Annual Data Report Bethesda, Maryland©2013 Cystic Fibrosis Foundation. Available online: http://www.cff.org/
- Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013;62:D117-26. [PubMed]
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43-54. [PubMed]
- Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001-2006. Heart 2009;95:312-7. [PubMed]
- Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012;125:113-22. [PubMed]
- Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011;37:665-77. [PubMed]
- Goldstein BS, Sweet SC, Mao J, et al. Lung transplantation in children with idiopathic pulmonary arterial hypertension: an 18-year experience. J Heart Lung Transplant 2011;30:1148-52. [PubMed]
- Hamvas A, Deterding R, Balch WE, et al. Diffuse lung disease in children: Summary of a scientific conference. Pediatr Pulmonol 2014;49:400-9. [PubMed]
- Kurland G, Deterding RR, Hagood JS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013;188:376-94. [PubMed]
- Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol 2009;12:253-74. [PubMed]
- Whitsett JA. Genetic disorders of surfactant homeostasis. Paediatr Respir Rev 2006;7 Suppl 1:S240-2. [PubMed]
- Shulenin S, Nogee LM, Annilo T, et al. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med 2004;350:1296-303. [PubMed]
- Nogee LM, Garnier G, Dietz HC, et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest 1994;93:1860-3. [PubMed]
- Nogee LM, Dunbar AE 3rd, Wert S, et al. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest 2002;121:20S-21S. [PubMed]
- Palomar LM, Nogee LM, Sweet SC, et al. Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure. J Pediatr 2006;149:548-53. [PubMed]
- Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med 2011;184:172-9. [PubMed]
- Mauad T, Dolhnikoff M. Histology of childhood bronchiolitis obliterans. Pediatr Pulmonol 2002;33:466-74. [PubMed]
- Fischer GB, Sarria EE, Mattiello R, et al. Post infectious bronchiolitis obliterans in children. Paediatr Respir Rev 2010;11:233-9. [PubMed]
- Nishio N, Yagasaki H, Takahashi Y, et al. Late-onset non-infectious pulmonary complications following allogeneic hematopoietic stem cell transplantation in children. Bone Marrow Transplant 2009;44:303-8. [PubMed]
- Uhlving HH, Buchvald F, Heilmann CJ, et al. Bronchiolitis obliterans after allo-SCT: clinical criteria and treatment options. Bone Marrow Transplant 2012;47:1020-9. [PubMed]
- Snell GI, Paraskeva M, Westall GP. Managing bronchiolitis obliterans syndrome (BOS) and chronic lung allograft dysfunction (CLAD) in children: what does the future hold? Paediatr Drugs 2013;15:281-9. [PubMed]
- Scully BB, Zafar F, Schecter MG, et al. Lung retransplantation in children: appropriate when selectively applied. Ann Thorac Surg 2011;91:574-9. [PubMed]
- Valapour M, Paulson K, Smith JM, et al. OPTN/SRTR 2011 Annual Data Report: Lung. Am J Transplant 2013;13:149-77. [PubMed]
- Liou TG, Adler FR, Cox DR, et al. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med 2007;357:2143-52. [PubMed]
- Thabut G, Christie JD, Mal H, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am J Respir Crit Care Med 2013;187:1335-40. [PubMed]
- Sweet SC, Aurora P, Benden C, et al. Lung transplantation and survival in children with cystic fibrosis: solid statistics-- flawed interpretation. Pediatr Transplant 2008;12:129-36. [PubMed]
- Adler FR, Aurora P, Barker DH, et al. Lung transplantation for cystic fibrosis. Proc Am Thorac Soc 2009;6:619-33. [PubMed]
- Aris RM, Routh JC, LiPuma JJ, et al. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am J Respir Crit Care Med 2001;164:2102-6. [PubMed]
- De Soyza A, McDowell A, Archer L, et al. Burkholderia cepacia complex genomovars and pulmonary transplantation outcomes in patients with cystic fibrosis. Lancet 2001;358:1780-1. [PubMed]
- Rama JA, Fan LL, Faro A, et al. Lung transplantation for childhood diffuse lung disease. Pediatr Pulmonol 2013;48:490-6. [PubMed]
- Khan MS, Heinle JS, Samayoa AX, et al. Is lung transplantation survival better in infants? Analysis of over 80 infants. J Heart Lung Transplant 2013;32:44-9. [PubMed]


