In vivo lung perfusion as a platform for organ repair in acute respiratory distress syndrome
Acute respiratory distress syndrome (ARDS) is characterized, according to the most recent Berlin definition, by acute onset of hypoxemia (PaO2/FiO2 ≤300) and bilateral pulmonary radiographic opacities, not primarily caused by left heart failure (1). This severe clinical condition culminates from a catastrophic inflammatory response following a direct or indirect acute insult to the lung. A recent global epidemiological investigation showed that there are greater than 3 million ARDS patients per year receiving mechanical ventilation in intensive care units. While intensive care capabilities have advanced significantly, mortality in ARDS patients has not significantly diminished and remains unacceptably high, exceeding 40% in those with the most severe form (2,3). Moreover, survivors have a high risk of experiencing post-traumatic stress disorder and functional deconditioning, which may significantly decrease their quality-of-life (4).
Currently, treatment of ARDS relies merely on supportive care of the respiratory function with mechanical ventilation delivered with limited pressure and volume settings, and prone positioning for patients with PaO2/FiO2 ≤150 (5). No specific pharmacological treatment has been found to improve outcomes, apart from administration of neuromuscular blockade during the first 48 hours after diagnosis. Even this is likely effective only by reducing the potential injurious effect of mechanical ventilation rather than through specific anti-inflammatory mechanisms (6). Therefore, there is a clear and pressing need to discover novel therapeutic options for this patient population.
Dr. Mehaffey and colleagues have developed a very innovative treatment modality to approach ARDS (7). Using intravenous lipopolysaccharide (LPS) injection to create an exogenous lung injury porcine model of ARDS, the authors have used in vivo lung perfusion (IVLP) as a rehabilitative therapy for inflamed lungs. This innovative application of IVLP was made feasible by supporting the gas exchange of animals with extracorporeal membrane oxygenation (ECMO). Based on the ex vivo lung perfusion (EVLP) technology, IVLP uses the identical circuit and balanced albumin and dextran-based perfusion solution, STEEN solution (8,9). EVLP was initially developed as a method to assess lung function and institute therapies in borderline lungs in the setting of lung transplantation (10). In addition, EVLP has been shown to maintain lungs without the deterioration typical of prolonged ischemic time. Accordingly, IVLP has been studied as a lung-protective method of achieving isolated lung circulation, initially for the purposes of delivering high dose chemotherapy to treat lung metastases. Building on the premise of achieving lung protection from this lung perfusion technology, the authors have explored using IVLP for rehabilitative purposes in acute lung injury.
In this study, IVLP applied to one lung was superior to ECMO alone, applied to the contralateral lung as an internal control, in treating LPS-induced lung injury. Specifically, the P/F ratio, compliance and wet-to-dry ratio all showed statistically significant improvement in the lung that underwent IVLP compared to the contralateral lung. Moreover, lung injury severity scores and inflammatory biomarkers including TNF-α, IFN-γ, and VCAM-1, were significantly lower in the lung treated with IVLP. As a result, over the 10-hour study period, 6 animals were successfully weaned from ECMO, demonstrating a significant clinical benefit from the IVLP approach. However, in analyzing this study in a larger context, it is important to consider several limitations of this work. Firstly, the injury model using LPS endotoxin administration mimics that of an exogenous lung injury since there was no direct pulmonary infection. Given that IVLP involves flushing out the lungs with perfusate, and that the experimental protocol involved a perfusate exchange after 2 hours, it is necessary to consider that some improvement may have been gained from reduction in LPS load in the lung. Accordingly, these results warrant confirmation using an endogenous lung injury model. Secondly, while this study provides an important proof-of-concept, the protocol used would not be immediately applicable to critically ill patients, considering the risk and the morbidity of the thoracotomy approach. Currently, IVLP techniques are in their infancy and would require major adjustments to be used in this population. As the authors have identified, a minimally invasive approach such as percutaneous cannulation and isolation would be vital to make such a treatment feasible. However, this is still a noteworthy challenge as it would require development of a novel advanced percutaneous system to cannulate and isolate the pulmonary artery and veins.
Overall, this study demonstrates a compelling proof-of-concept for an innovative approach for treatment of lung injury in ARDS. IVLP provides an intriguing platform for treatment of ARDS because it allows complete flexibility and control over the targeted organ. This flexibility allows for several possible advantages and benefits. Firstly, IVLP removes the blood from the organ environment. By decreasing the exposure to circulating toxins, white blood cells, and inflammatory mediators, the inflammatory response may be mitigated as shown in this study. Another interesting application involves the fact that it is possible to adjust perfusion and ventilation settings during IVLP with the aim of optimizing V/Q matching. Ventilation settings that are optimal for rehabilitating damaged lungs are already being investigated and it may be possible that control over perfusion can similarly be of benefit.
It is worth mentioning that EVLP has also been explored as an alternative treatment platform to IVLP, by the same group, as a platform for rehabilitation of the lung (11). With ECMO supporting the vital function of the patient, it is similarly possible to provide targeted treatment to the lung on EVLP. In addition, removing the lung from the toxic environment for EVLP may be appealing to separate the lung from ongoing inciting exposures to lung injury (12). However, despite this appeal, EVLP would be more problematic compared to IVLP, as it requires a more invasive approach involving removal and re-implantation of the lung. This would result in the potential for complications from the vascular and bronchial anastomoses as well as disruption of the bronchial circulation and innervation, which are important in maintaining homeostasis of the organ. While more work is needed to explore these approaches, it stands to reason that IVLP, as a less invasive method that preserves the lungs neurohormonal, lymphatic and bronchial circulatory systems, would provide several long-term benefits and mitigate morbidity associated with treatment.
Future directions
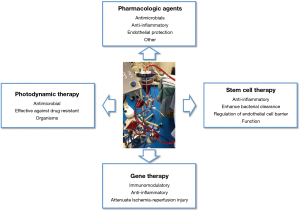
IVLP provides a treatment platform that has the unique characteristic in that it is isolated from the systemic circulation, thereby allowing for the rapid delivery of therapies that would not be suitable in the systemic circulation. This opens the door for various innovative and exciting applications that are on the horizon (Figure 1). Several intriguing and novel modalities that are being explored include pharmacologic agents, gene therapy, stem cell therapy and photodynamic therapy (PDT), amongst others.
Pharmacologic agents are the simplest conceptual application as the isolated approach prevents risks from systemic toxicity and adverse events, while also improving the pharmacokinetics by excluding the agent from passing through the liver or kidneys. Antimicrobials are an attractive potential option as certain agents, that have previously been avoided due to significant toxicities, can be safely used. Alternatively, recent work has been done on developing agents that counteract the endothelial permeability characteristic of inflammatory-mediated pulmonary edema. One such example, Vasculotide, is an angopoeitin-1 mediated Tie-2 agonist, that has shown the ability to stabilize endothelial function and reduce lung permeability in both in vitro and in vivo studies of acute lung injury (13,14). One study showed significant reduction in mortality and pulmonary edema in a murine model of severe influenza up to 72 hours after initial infection. This agent, alone or in combination with antimicrobials, may provide a new direction of investigation with lung perfusion. Similarly, there has been recent interest in exploring the use of α1-Anti-trypsin (A1AT) for its anti-inflammatory effect and its ability to attenuate ischemia-reperfusion injury. A1AT has traditionally been used to treat emphysema in A1AT deficient patients. Of interest, a recent study showed that A1AT treatment during EVLP in a pig donor lung model of 24 hours prolonged cold ischemic time improved function and reduced injury compared to standard EVLP (15). Overall, pharmacologic agents represent a simple yet effective treatment modality to be added with lung perfusion, that may provide significant advantages in ARDS lungs.
The use of stem cell therapies to repair damaged organs has been a field of growing research for decades. For the lung, mesenchymal stromal cells (MSCs) have been explored in reducing lung injury in multiple settings (16,17). MSCs have further been studied in the setting of EVLP for preventing ischemia-reperfusion injury and chronic lung allograft dysfunction in lung transplantation. Our group has shown that MSC instillation in the EVLP circuit resulted in significant uptake of MSCs in the lung parenchyma and was associated with reduction in pro-inflammatory cytokines (18). More relevant to the ARDS setting, one group has shown that MSC instillation promoted recovery in lungs injured by acute E. coli infection by reducing inflammation, restoring alveolar fluid clearance and aiding in bacterial clearance (17,19). These results provide a compelling basis for extrapolating to a sepsis induced lung injury in an IVLP setting.
Gene therapy provides another exciting potential modality that is attractive in an isolative lung perfusion setting. There has been significant interest in exploring gene therapy application in transplant settings to attenuate ischemia-reperfusion injury. Many of these studies have looked at gene therapy to overexpress anti-inflammatory cytokines. However, delivery of gene therapy by viral vectors can result in a significant inflammatory response, counter-productive to the intended mechanism (20). Lung perfusion accordingly becomes an attractive platform for gene therapy since the host immune system is largely separated during lung perfusion, thereby lessening vector-associated inflammation. Previous studies from our group have shown that Il-10 gene therapy during EVLP is superior to in vivo delivery in attenuating ischemia-reperfusion injury and its safety and efficacy in improving donor lung function. It accordingly stands to reason that IVLP instillation will be similar to the EVLP environment, although further study is required to confirm this (21-23). In the ARDS setting, preventing the pro-inflammatory cascade characteristic of this syndrome through gene therapy provides an intriguing theoretical approach that could reduce the severity and duration of critical illness.
PDT is a modality that benefits from the flexible environment in organ perfusion settings. PDT requires the activation of a drug, called a photosensitizer, by light of a specific wavelength to produce reactive oxygen species for tumoricidal or antimicrobial effect. Relevant to the treatment of ARDS from pneumonia, there are numerous studies describing antimicrobial PDT in various models including gram-positive and gram-negative bacteria, yeast, fungi, viruses and parasites (24,25). Moreover, PDT is not sensitive to the traditional development of resistance as it does react with any one specific zone on its target. However, the use of PDT on organs has traditionally been limited by the inability to penetrate light deeply into tissue, largely due to the effect of hemoglobin which naturally absorbs a significant proportion of light (26,27). In the organ perfusion setting, hemoglobin is washed out and acellular perfusate is circulated instead, theoretically allowing greater light penetration and resultantly better uptake of the PDT effect.
Perfusion technologies are triggering a paradigm shift in the way that critically ill patients are treated. The expansion in the use of ECMO has made it possible to consider treatments, such as IVLP, that would previously have been impossible to use while the lungs are required to perform gas exchange. The work by Dr. Mehaffey and colleagues is important because it represents this change in perspective. Building upon these findings, exciting frontiers are available to explore the use of ECMO, IVLP and percutaneous perfusion technologies in the support and rehabilitation of patients who previously had no hope of recovery.
Acknowledgements
None.
Footnote
Conflicts of Interest: M Cypel has received research support from Xvivo Perfusion and is a consultant for Lung Bioengineering. M Cypel is founding shareholder of Perfusix Canada and XOR labs Toronto. The other authors have no conflicts of interest to declare.
References
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 2009;179:220-7. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293-304. [Crossref] [PubMed]
- Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;195:1253-63. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Mehaffey JH, Charles EJ, Schubert S, et al. In vivo lung perfusion rehabilitates sepsis-induced lung injury. J Thorac Cardiovasc Surg 2018;155:440-8.e2. [Crossref] [PubMed]
- dos Santos PR, Iskender I, Machuca T, et al. Modified in vivo lung perfusion allows for prolonged perfusion without acute lung injury. J Thorac Cardiovasc Surg 2014;147:774-81: discussion 781-2.
- Reck Dos Santos P, Sakamoto J, Chen M, et al. Modified In Vivo Lung Perfusion for Local Chemotherapy: A Preclinical Study With Doxorubicin. Ann Thorac Surg 2016;101:2132-40. [Crossref] [PubMed]
- Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319-25. [Crossref] [PubMed]
- Mehaffey JH, Charles EJ, Sharma AK, et al. Ex Vivo Lung Perfusion Rehabilitates Sepsis-Induced Lung Injury. Ann Thorac Surg 2017;103:1723-9. [Crossref] [PubMed]
- Cypel M, Waddell T, Singer LG, et al. Bilateral pneumonectomy to treat uncontrolled sepsis in a patient awaiting lung transplantation. J Thorac Cardiovasc Surg 2017;153:e67-9. [Crossref] [PubMed]
- Sugiyama MG, Armstrong SM, Wang C, et al. The Tie2-agonist Vasculotide rescues mice from influenza virus infection. Sci Rep 2015;5:11030. [Crossref] [PubMed]
- Gutbier B, Jiang X, Dietert K, et al. Vasculotide reduces pulmonary hyperpermeability in experimental pneumococcal pneumonia. Crit Care 2017;21:274. [Crossref] [PubMed]
- Lin H, Chen M, Tian F, et al. α1-Anti-trypsin improves function of porcine donor lungs during ex-vivo lung perfusion. J Heart Lung Transplant 2018;37:656-66. [Crossref] [PubMed]
- van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med 2009;180:1131-42. [Crossref] [PubMed]
- Lee JW, Krasnodembskaya A, McKenna DH, et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med 2013;187:751-60. [Crossref] [PubMed]
- Mordant P, Nakajima D, Kalaf R, et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J Heart Lung Transplant 2016;35:1245-54. [Crossref] [PubMed]
- Park J, Kim S, Lim H, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax 2019;74:43-50. [PubMed]
- Martins S, de Perrot M, Imai Y, et al. Transbronchial administration of adenoviral-mediated interleukin-10 gene to the donor improves function in a pig lung transplant model. Gene Ther 2004;11:1786-96. [Crossref] [PubMed]
- Cypel M, Liu M, Rubacha M, et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med 2009;1:4ra9. [Crossref] [PubMed]
- Yeung JC, Wagnetz D, Cypel M, et al. Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol Ther 2012;20:1204-11. [Crossref] [PubMed]
- Machuca TN, Cypel M, Bonato R, et al. Safety and Efficacy of Ex Vivo Donor Lung Adenoviral IL-10 Gene Therapy in a Large Animal Lung Transplant Survival Model. Hum Gene Ther 2017;28:757-65. [Crossref] [PubMed]
- Antimicrobial Photodynamic Therapy: A New Therapeutic Option to Combat Infections. Available online: http://omicsonline.org/open-access/antimicrobial-photodynamic-therapy-a-new-therapeutic-option-to-combat-infections-2161-0703.1000158.php?aid=33088
- Sharma SK, Dai T, Kharkwal GB, et al. Drug discovery of antimicrobial photosensitizers using animal models. Curr Pharm Des 2011;17:1303-19. [Crossref] [PubMed]
- Sandell JL, Zhu TC. A review of in-vivo optical properties of human tissues and its impact on PDT. J Biophotonics 2011;4:773-87. [Crossref] [PubMed]
- Wang HW, Zhu TC, Putt ME, et al. Broadband reflectance measurements of light penetration, blood oxygenation, hemoglobin concentration, and drug concentration in human intraperitoneal tissues before and after photodynamic therapy. J Biomed Opt 2005;10:14004. [Crossref] [PubMed]