
Outcomes and factors associated with early mortality in pediatric and neonatal patients requiring extracorporeal membrane oxygenation for heart and lung failure
Introduction
Survival rates and life expectancy after use of extracorporeal membrane oxygenation (ECMO) and extracorporeal life support (ECLS) for severe pulmonary and cardiac failure is rapidly increasing. Nevertheless, mortality and morbidity after surgical repair for complex congenital heart defects and severe cardiopulmonary failure on ECMO support remain high. Since its first successful application in a newborn in 1971, ECMO treatment was evolved to a valuable tool for therapy of severe cardiac and respiratory failure (1,2). The common clinical scenarios for use of veno-arterial (va) ECMO in neonatal and pediatric patients include failure to wean off cardiopulmonary bypass (CPB) following cardiac surgery, refractory cardiac arrest of any etiology (eCPR—extracorporeal cardiopulmonary resuscitation), and circulatory failure despite exhaustion of conservative medical therapy options (3-7). The most common indications for utilization of veno-venous (vv) ECMO in pediatric patients are severe respiratory failure (i.e., viral pneumonia (influenza and parainfluenza, bacterial pneumonia, status asthmaticus, acute respiratory distress syndrome (ARDS) due to sepsis, aspiration pneumonia etc.) and in fewer cases—bridge to lung transplantation (8-11). In neonatal patients veno-venous ECMO remains an instrumental choice for treatment of congenital diaphragmatic hernia (CHD), meconium aspiration syndrome (MAS) and persistent pulmonary hypertension of the newborn (PPHN). The use of vvECMO for neonatal patients is decreasing in the last years most likely due to advancements in medical management and ventilation strategies (5,12-15).
The contraindications for neonatal ECMO therapy are divided in absolute (grade III–IV intraventricular hemorrhage (IVH), uncontrolled coagulopathy, lethal chromosome abnormality, irreversible organ damage, unless ECLS is being used as a bridge to transplant) and relative (mechanical ventilation >10–14 days, weight <2 kg, and gestational age <34 weeks). The use of ECMO for cardiac disease has grown over time with an ever-shrinking numbers of contraindications involving the sickest and complicated babies and children. Today approximately 2–5% of neonatal and pediatric patients after cardiac surgery may require ECMO for cardio-respiratory support (16-21).
The main complications of ECMO therapy are mechanical complications (oxygenator failure, pump malfunction, pump and oxygenator thrombosis etc.), surgical site bleeding with the need for re-exploration, intracerebral hemorrhage, hemolysis, acute kidney injury, bloodstream infections and other complications (3,22). Neurological issues also remain one of the common and serious complications in patients receiving ECMO-therapy (1,11). Despite their improving efficacy, ECMO and ECLS utilization varies between different institutions based on various factors, such as experience, logistics, and manpower availability. Skilled personnel and appropriate financial means remain the most important prerequisites for socio-economic aspects of this expensive therapy (3,17,23-25).
In the current report, we summarize our institutional experience with the main focus on presenting outcomes and factors after using ECMO and ECLS in our surgical pediatric population including short- and long-term survival.
Methods
Data from 45 neonatal and pediatric patients who required ECLS/ECMO for cardiac or lung support from 2008 to 2016 were retrospectively obtained from our institutional electronic medical records and from patient charts. The institutional review board of Cologne University approved this study and waived the need for individual patient consent. All procedures followed were in accordance with ethical standards, current guidelines and Helsinki Declaration. In 41 cases (91%) a vaECMO, was implemented whereas 4 patients (9%) received vvECMO treatment for respiratory failure. Age groupings of all patients in the present study were defined as neonates (<1 month), infants (>1 month; <2 years), toddlers (2–4 years), and elderly children (>4 years; <18 years). For the better differentiation of ECMO complications and mortality the age groupings stated above were sought to be most appropriate. The majority of Norwood I, Glenn procedures and other single ventricle (SV) palliation procedures have to be performed in neonatal ages, so that the neonates whom underwent this procedures and need ECMO support after surgery constitute specific patient cohort to investigate. Furthermore, as described in several previous studies the neonates and infants has specific miniaturization prerequisites to the ECMO equipment, which also makes them susceptible to the negative ECMO events such as cerebral haemorrhage and another major bleeding complications. According to these facts the patients included in this study were divided into age groupings mentioned above.
The primary end point of the present study was survival to discharge and long-term survival free from neurological impairment. Follow-up was obtained from an out-patients clinic reports. Only one single patient was not followed up due to lost contact and therefor excluded from long-term observation. The Pediatric Cerebral Performance Category (PCPC) was used to evaluate gross neurological outcomes in survivors. Statistics was performed using Student t-test and Mann-Whitney U test for continuous variables and Chi-square test for categorical variables. The Fisher exact test was performed when minimum expected count in >20% of cells was <5. Univariate and bivariate analysis was performed to address predictors of outcome. Cut off values were chosen with regard to means/medians and Log-Rank test via receiver operating curves (ROC). Kaplan-Meier survival analysis was used to address mid- and long-term survival. A P value <0.05 was considered significant. All statistical analysis was performed utilizing SPSS Version 25.0 (IBM Corp., Chicago, IL, USA).
Cardiac support (ECLS or vaECMO)
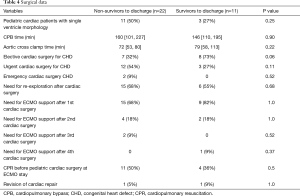
In 33 cases ECLS was implanted following cardiac surgery for congenital heart disease (CHD), whereas in 24 cases (73%) ECLS was implanted following first, in 6 (18%) following second, 2 (6%) following third and 1 (3%) case following fourth cardiac surgery for CHD. For patients, who failed weaning off CPB (n=19, 58%) a central vaECMO approach using previously inserted bypass cannulas was applied. Only one single patient underwent cannulation via femoral vessels for vaECMO support during cardiac surgery (Ross procedure). The central cannulation was also preferred for children requiring ECLS in the neonatal intensive care unit (NICU) or pediatric intensive care unit (PICU) in the immediate postoperative period (n=12, 36%). Only a single vaECMO from PICU was established via neck vessels in a child with empyema with pericarditis.
Cardiac surgery was defined as urgent surgery when the child undergone cardiac surgery within 5 days and as emergent surgery when patient was operated on within the next few hours after admission to hospital or after deployment of diagnosis. For elective surgery children were scheduled weeks or months before surgery. The rest of patients supported using ECLS (n=8) were treated by means of extracorporeal cardiopulmonary resuscitation (eCPR) following cardiac arrest (eCPR group). Femoral approach was applied in 6 children outside of our hospital with subsequent transportation to our department, whereas central cannulation was performed in 2 children with cardiac arrest requiring CPR in our institution followed refractory cardiac arrest after pediatric cardiac interventions.
Pulmonary support (vvECMO)
Four patients received femoral cannulas for vvECMO support, whereas two patients had additional neck cannulas for vvECMO support. Also, two patients had second ECMO support during the same admission, whereas an additional patient had to be upgraded to vaECMO support due to subsequent cardiac failure.
Equipment
A standard ECMO circuit consisting of a centrifugal pump head (Jostra; Maquet Inc., Rastatt, Germany) and albumin/heparin coated membrane oxygenator (Medos Hilite; Xenios AG, Heilbronn, Germany) were used. A disposable heat exchanger was used to maintain constant blood temperature in the circuit. The priming volume of the circuit depended on the surface area of the oxygenator and patient’s weight. In cases of extreme emergency ECMO circuit was primed with crystalloids (Plasmalyte 148; Baxter Corp.; Canada, Toronto). If packed red blood cells (PRBCs) and fresh frozen plasma’s (FFPs) were immediately available, priming with blood products was preferred. The circuit required an average priming volume of 250 mL for an infant or 400 mL for older children. The excess crystalloid was removed by a manual exchange transfusion method. Each ECMO used in the operating room was implanted following an attempt to wean off CPB and reversal of heparinization to improve hemostasis. Hematocrit value of greater than 35% was targeted in all cases, whereas hematocrit value of 45% was the goal before discontinuing ECMO, especially in cyanotic patients.
The ECMO flow was adjusted to restore and maintain an adequate tissue perfusion pressure with the goal of reversal of metabolic acidosis and maintenance of mixed venous saturation above 70%. After ECMO initiation unfractionated heparin was continuously infused. An activated clotting time (ACT) was checked and adjusted every 4 h. Prothrombin time (PT), activated partial thromboplastin time (aPTT) and platelet count were monitored once a day, if there was no active bleeding tendency. Other important laboratory parameters, such as blood count, cardiac sensitive enzymes, liver function enzymes, blood urea nitrogen (BUN), creatinine clearance, glomerular filtration rate (GFR), bilirubin, and CRP were also monitored and documented before and after ECMO implantation on daily basis. Arterial blood gas was monitored every hour, whereas pre ECMO blood gas parameters were obtained directly before ECMO cannula placement and subsequent measurements were performed directly after ECMO insertion, as well as 3, 6, 12, 24, and 72 h after ECMO implantation). When on full ECMO flow, inotropic support was kept at the lowest possible level. Minimal inotropic support (2–3 mcg/kg/min dobutamine, 0.1–0.25 mcg/kg/min milrinone) was continued to left ventricular vent with the view to preventing left ventricular distension in nearly all cases. The ventilatory support was stipulated to a minimal PEEP of 5–8 mmH2O, Pinsp of 20 mmH2O, and FiO2 of 40% during full ECMO flow. After commencement of ECMO weaning the vasoactive and ventilatory support was increased stepwise. The gas flow and blender on the ECMO circuitry were adapted incessantly to keep blood gas values in normal range. Diuretics were administered, if urine output declined below 2 mL/kg/h. Hemodialysis or peritoneal dialysis was used in patients with renal insufficiency or renal failure.
The timing of ECMO weaning and disconnection depended on individual clinical scenario, progress of myocardial recovery, hemodynamic stability, and surgical or interventional suitability. The separation from ECMO support was completed in satisfactory hemodynamic situations with low to moderate doses of catecholamines. Decannulation was also considered for patients with insufficient improvement of cardiac or pulmonary function after reasonable support duration or by futility of medical care continuation, such as severe brain damage or significant uncontrolled bleeding. Withdrawal of therapy was suggested to be appropriate when ongoing benefit became exceedingly unlikely and ongoing ECMO support was only associated with occurrence of additional morbidities and complications without the potential for achieving disease reversal, organ replacement, or adequate symptom management and acceptable quality of life. Neurologic impairment was defined as any major cerebrovascular event, such as stroke, IVH, cerebral infarction, seizures, hypoxic/anoxic brain injury, clinical brain death, or severe cerebral edema with protrusion. By any clinical conspicuousness of neurologic complications cerebral sonography was performed followed by cerebral computed tomography (cCT) and/or electroencephalography (EEG). Sepsis refers to clinical signs of systemic inflammatory response syndrome (SIRS) with verified bloodstream infection. If the infect was not culture-proven it was defined as SIRS.
Primary ECMO related mechanical complications included pump thrombosis, insertion site bleeding, and hemolysis. Further frequent complications during mechanical support such as limb ischaemia or embolia, peritoneal dialysis, tracheostomy, infection, type 2 heparin induced thrombocytopenia (HIT), pulmonary hemorrhage, and malignant arrhythmias, such as ventricular tachycardia (VT), junctional ectopic tachycardia (JET), complete atrioventricular (AV) block with or without the need for pacemaker implantation were furthermore noticed.
Results
Median age at ECMO implantation was 128 [14, 1,813] days, median weight of patients was 5.4 [3.3, 12] kg. A total of 18 (40%) patients died on ECMO, whereas 7 (16%) patients died within 24 h after withdrawal of ECMO. A total of 20 (44%) patients were successfully weaned off ECMO (survived >24 h after ECMO explantation), whereas 15 (33%) of them survived to discharge (Table 1). The first 24 h were a crucial phase in respect to survival after ECMO discontinuation (P=0.001). Female patients showed a statistical non-significantly decreased odds of survival on ECMO compared to male patients [OR 0.46 (0.13–1.6); P=0.22]. Also in survival to discharge the difference between male and female patients did not reach statistical significance between two groups (P=0.19, Table 2). Median duration of ECMO support was 3 [2, 5] days (range, 1–17 days). Beneficial duration of support was limited by 10 days (no survivors after 10 days of support continuation were documented).
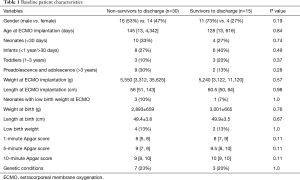
Full table
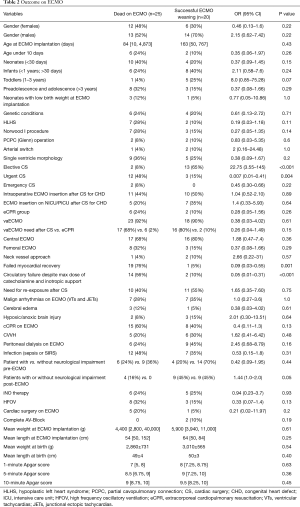
Full table
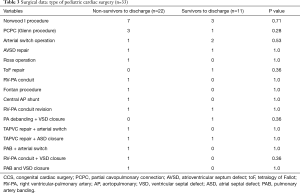
Totally 10 patients were diagnosed with chromosomal abnormalities in this study. In two patients with vaECMO support 1p36 deletion syndrome was found, in another two patients Down syndrome was present. Other 6 patients were presented with CHARGE syndrome; 16p11.2 deletion; congenital disorder of glycosylation; Marcus Gunn Jaw-Winking syndrome; VACTERL association, and Noonan syndrome. There was no statistical significant difference in outcome on ECMO and in-hospital mortality of this specific patient cohort compared to patients without genetic conditions, also patients with genetic disorders had non-significantly decreased odds for ECMO weaning [OR 0.32 (0.07–1.48), P=0.134]. There was a wide range of indications for ECMO therapy in our patient cohort (Figures 1,2). Surgical procedures were described in Table 3. Primary diagnoses for surgery were hypoplastic left heart syndrome (HLHS) in 9 patients, dextro transposition of the great arteries (dTGA) in 3, double outlet right ventricle (DORV) in 3, tetralogy of Fallot (ToF) in 2, total anomalous pulmonary venous connection (TAPVC) in 2, atrioventricular septum defect (AVSD) in 2 patients, and congenitally corrected TGA (cc-TGA) in 1 patient, respectively. Other combined and mixed inborn pathologies contributed to the perioperative ECMO group were depicted in Figure 1. Median [IQR] CPB time was 146 [105, 207] min with median [IQR] aortic cross clamp time of 74 [55, 87] minutes. The most common surgical procedures associated with the need for ECLS after congenital heart surgery constituted Norwood I, PCPC (Glenn procedure) and arterial switch operation patients (Table 3). Of all patients with CHD a SV repair was performed in 14 (42%) and biventricular repair in 19 (58%) patients (P=0.25). SV repair was associated with decreased odds of survival on ECMO (Table 2). Children with HLHS had low odds of successful ECMO weaning [OR 0.19, 95% CI (0.03–1.18), P=0.11]. Elective surgery was associated with significantly better outcome after ECMO weaning, whereas urgent and emergent surgery were highly associative with poor outcome (P<0.001). Both patients who required emergency surgery died after ECMO discontinuation. Elective cardiac surgery procedures showed a trend towards favorable outcome in regard to in-hospital survival as well. Two patients (6%) required surgical revision after previous cardiac repair, whereof one survived to discharge (Table 4).
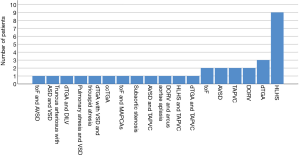
Full table
Full table
Totally 6 patients (13%) underwent various cardiac surgical procedures after commencement of ECMO therapy. Surgical procedures on ECMO were related to low odds of survival on ECMO [OR 0.21, 95% CI (0.02–11.97), P=0.2]. ECMO insertion in operating theatre was associated with higher odds of mortality on ECMO compared to ECMO insertion at NICU or PICU [OR 1.4, 95% CI (0.33–5.93), P=0.64]. Also, central ECMO cannulation approach was strongly associated with surgical revision compared to peripheral ECMO cannulation (P=0.009).
A total of 14 neonates (<1 month of age, 31%) who needed ECMO support were included in our study, whereof 4 patients (29%) were successfully weaned off ECMO support and survived to discharge. Further 14 patients were infants (31%), 6 toddlers (13%), and the remaining 11 patients were older (24%). Infants and toddlers showed higher odds of ECMO survival [OR 2.11, 95% CI (0.58–7.6), P=0.24 and OR 8.0, 95% CI (0.85–75.28), P=0.07]. The children in preadolescence and adolescence were at high risk not to survive after ECMO separation (Table 2). A total of 4 neonatal ECMO patients with low birth weight (<2,000 g) were identified in the present study. All of these patients required urgent surgery for CHD (three patients for Norwood I, and one patient for arterial switch procedure). Two of them died on ECMO, whereas the remaining two patients were weaned off ECMO support with only a single survival to discharge without neurological deterioration. Furthermore, there were two neonates with low birth weight who gained weight to a normal range and needed ECMO support few months later, following congenital cardiac surgery (CCS) with one death and one survival to discharge. Neonates, especially those with age under 10 days, were at high risk of mortality on ECMO [survival OR 0.37, 95% CI (0.09–1.45) and 0.35, 95% CI (0.06–1.97)].
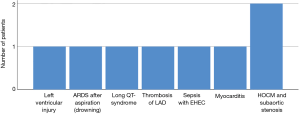
In 8 children ECMO was used as eCPR to augment the conventional CPR (cCPR) (Figure 2). Median cCPR time was 61 {[22, 115] range 15–210} min. Of them, in two patients eCPR was utilized as life-saving measure after cCPR following pediatric cardiac interventions for HOCM with concomitant subaortic stenosis. One patient with severe sepsis was supported with eCPR due to refractory circulatory failure. The responsible pathogen was Enterohemorrhagic E. coli triggering hemolytic uremic syndrome. Another patient suffered a stabbing injury and underwent emergent surgery in form of left ventricular suture under ECMO support. In addition one patient received ECMO after he drowned in cold water with subsequent cCPR for more than 2 h. The prognosis of last two mentioned children was dismal and unfortunately both patients died on ECMO. Other primary disease entities preceding initiation of ECMO/ECLS in the eCPR group were long QT-syndrome (n=1), myocardial dysfunction due to myocarditis with the history of scarlet fever (n=1) and myocardial infarction due to acute thrombosis of left artery descending (LAD, n=1). The patient who underwent cCPR due to long QT-syndrome subsequently underwent a long-term left ventricular assist device (LVAD) implantation and died shortly thereafter as a result of a large stroke und right ventricular failure. The patient with myocarditis was placed on vaECMO mode because of viral damage to myocardium. After myocardial recovery the patient survived to discharge. In general eCPR was associated with low odds of survival on ECMO therapy [OR 0.28, 95% CI (0.05–1.56), P=0.26]. Of overall, 41 vaECMO cases eCPR group was also associated with adverse outcome compared to ECMO cases after CCS [OR 0.26, 95% CI (0.04–1.49), P=0.15]. Survival to discharge in this group accounted for merely 22% (n=2). Only 1 child with eCPR after 15 min of cCPR had no neurological complications; regrettably all other but one patient with distinctly longer cCPR time died thereafter. The last mentioned child retained minimal motoric residues.
From vvECMO population, one patient was admitted to our ICU with emergency indication for vvECMO due to complex respiratory tract obstruction, which was not responsive to numerous bronchoscopies and recruitment maneuvers. Another patient developed a profound sepsis and ARDS with a number of bacterial and fungal pathogens after chemotherapeutical treatment for neuroblastoma. A further patient had also met vvECMO criteria due to severe ARDS. Another female patient had empyema with pericarditis and experienced severe pulmonary failure requiring vvECMO support. From this patient cohort the last two mentioned patients survived to discharge.
In general, the patients with vaECMO mode had lower odds of survival compared to vvECMO mode [OR 0.38, 95% CI (0.03–4.02), P=0.61]. Central ECMO was advantageous with higher odds of survival compared to peripheral ECMO [OR 1.88, 95% CI (0.47–7.4), P=0.36].
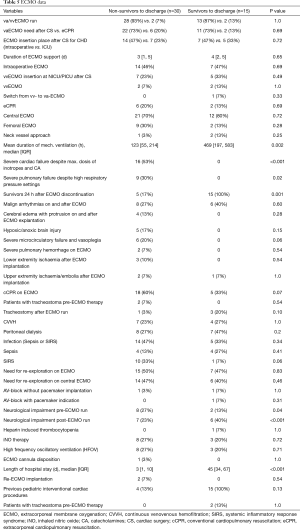
Failed myocardial recovery (P=0.001), profound circulatory failure despite high dose of catecholamines (P<0.001), neurological impairment pre-ECMO and post-ECMO (P=0.04 and P<0.001, respectively), severe pulmonary failure despite high respiratory pressure settings (P=0.02) were associated with mortality on ECMO (Table 5).
Full table
In total, repeated ECMO support during the same admission was required in two cases from the entire cohort, whereof termination of life support was necessary in both cases due to futile prognosis. All patients who developed cerebral edema with protrusion, hypoxic/anoxic brain injury, severe pulmonary hemorrhage (unresponsive to medical efforts to achieve hemostasis), and lower extremity ischemia also died during their hospital stay. SIRS or sepsis, cCPR on ECMO, continuous venovenous hemofiltration (CVVH) and/or peritoneal dialysis, iNO therapy and high frequency oscillatory ventilation (HFOV) on ECMO, and pre ECMO presence of tracheostomy were more frequently present in non-survivor group, however, it did not reach statistical significance (Table 5). One single patient developed ECMO flow instability as result of cannula disposition directly after cannulation and died on support.
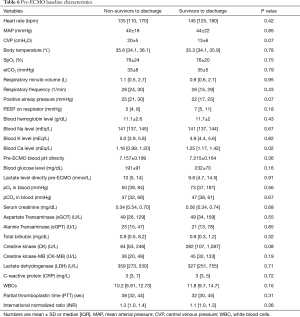
Further characteristics including vital parameters, such as heart rate, mean arterial pressure (MAP), central venous pressure (CVP), body temperature, partial oxygen pressure (pO2), endexspiratory carbon-dioxide (etCO2), respiratory settings, blood pH, hemoglobin, serum levels of ionic natrium (Na+), potassium (K+), hydrogen carbonate (HCO3–), lactate and blood sugar did not vary between both groups before ECMO application (Table 6). However, there was a trend towards higher respiratory positive airway pressure (PAW pressure) in non-survivors (P=0.07). Pre ECMO ventilation with positive airway pressure higher than 25 mmHg was shown to be associated with an eminent increase in hospital mortality in the present study reflecting significant lung injury (P=0.03).
Full table
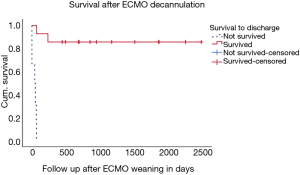
Due to obvious factors, children who survived had significantly longer duration of mechanical ventilation and longer total hospital stay than children who deceased (P value 0.002 and <0.001 respectively). During follow-up after hospital discharge {median [IQR] 848 [436, 1,827], range 26–2,439 days} only two children died. One of them had seizures with subsequent aspiration and required cCPR without any responsiveness to medical actions. The second child died due to severe major bleeding complications ensuing from thrombolytic therapy for tricuspid valve thrombosis. Eight ECMO survivors have been doing well with good neuropsychological development (Figure 3). There was no change in PCPC scores from baseline 1–2 in these 8 ECMO survivors. Other 4 children had PCPC score >3 at hospital discharge.
Discussion
There has been an exponential increase in ECMO/ECLS utilization for cardiac support in pediatric patients following congenital heart surgery after its first reported use by Baffes in 1973. On the other hand ECMO gives health-care specialists the opportunity to support cCPR and allows for resuscitation of a significant proportion of patients who would otherwise decease (4,26). vvECMO mode had been used in pediatric population for severe respiratory failure for more than 40 years with relatively stable numbers of patients supported and marked increase in case number by Influenza A H1N1 outbreak in 2009. vvECMO was associated with encouraging results in this patient cohort (1,13).
In the present study overall survival to discharge (33% of the entire patient cohort) was comparable to other studies in published literature, however better results were also described for neonatal and pediatric population in several reports (3,19,21,27,28). This can be attributed to the trend of ECMO use in more critically ill patients included in this study, compared to previously analyzed cohorts. The population of this study contains a large number of neonatal and pediatric patients with genetic abnormalities particularly with additional serious comorbidities. Only fewer previous studies investigated potential association between such genetic conditions and outcomes on ECMO (21,29,30). Also, in the majority of eCPR patients in this study ECMO was implanted little time after cCPR commencement possibly having an additional negative impact on our outcome (31-33). In this context the key to survival may be timely recognition, rapid launching of cCPR protocol and strict timing of ECMO implantation. However, due to heterogeneity of locations, availability of qualified staff and equipment, logistical organization represents a huge challenge for mobile ECMO teams in cases of eCPR. Therefore, improvements in mobility and organizational strategies in eCPR should be the focus for future research.
In this study male gender had decreased odds of ECMO mortality. There are controversial data in literature with the evidence on the impact of race and gender on ECMO survival. Several studies pointed out on a number of effects (29,34,35) related to race and gender, whereas other studies could not show significant relationship (36,37). In this view it is rather difficult to interpret potential protective effects of male gender against ECMO complications that were shown in our study. Thus, further investigations are also required in this field.
Weaning off ECMO is acknowledged as a complex process and occasionally can be extremely challenging as it is enormously difficult to accurately estimate the recovery of myocardial function. Also, termination of ECMO support is required in a substantial proportion of patients with futile prognosis. Seven of 19 neonates/infants in the present study died within first 24 h after ECMO weaning. The validity of echocardiographic monitoring in terms of myocardial recovery is associated with several limitations due to partially subjective assessment and depends on experience of examining clinician. In this matter the new strategies of ECMO weaning including the strategy of reversing the ECMO blood flow to conduct an effective stress test for the heart, thereby assessing the readiness for ECMO explantation, may be of further assistance in the decision making process (38). Heart transplantation and short-/long-term VADs represent alternative strategies in cases of unsuccessful weaning (23,27,28). However, seven patients who did not survive the first 24 h after ECMO explantation mentioned above were not considered eligible for such alternative treatment strategies for various reasons. Also, current shortage in organ availability, VAD size and complex anatomy and physiology of neonates/infants present further problems for these approaches (5).
According to several publications, survival is inversely correlated with ECMO duration, especially in cases of support for more than 7 days. Prolonged duration of ECLS/ECMO can indicate patients’ dependency on ECMO and might be a surrogate for possibly irreversible underlying illness (19,27,29,35). Inability to wean off and explant ECMO after 10 days of support with no survivors in the present study validates it as a potential predictor for higher mortality.
In this study the children who underwent Norwood procedures created the largest group of patients requiring ECMO support after CCS. Recent studies identified the need for ECMO after Norwood procedure as a prognostic factor associated with early heart failure (39,40). Particularly staged SV palliation procedures represent an increasing proportion of neonatal ECMO cases (22,39). Historically in some centers ECMO was routinely implemented after surgical repair of HLHS and its variants (40,41). In this regards Mascio et al. analyzed Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD) data for strategies in mechanical circulatory support (MCS) use and outcomes across participating centers in this society. Greatest use of MCS was not surprisingly found for SV palliative procedures and complex biventricular repairs (42). This finding also corroborates results in the present patient cohort. Volume overloading, cyanotic diseases with decreased pulmonary blood flow and increased pulmonary congestion force acute surgical interventions conceivably resulting in high mortality in cases of urgent and emergent surgery in SV patients as well as patients with dTGA and TAPVC (19,21).
Chrysostomou et al. showed that increasing complexity of cardiac defects, other associated anomalies and delays in ECMO/ECLS initiation might be potential reasons for the relatively lower survival (43). All children with CHD undergoing CCS in our study had higher RACHS-1 scores (>3) comprising more than 2/3 of all patients in the present study. Timely intraoperative ECMO implantation was shown to be associated with favorable survival in many studies pleading for early possible institution of ECLS/ECMO following correction of CHD and difficulties to wean off CPB. In contrast to these results 11 of 20 intraoperative ECMO circuits commenced in operating theatre compared to 7 of 12 ECMO circuits instituted on ICU were successfully weaned off in the present study.
Patients who underwent central ECMO cannulation needed more frequent re-explorations than patients with peripheral ECMO. This is obviously attributed to the fact that the cannulae in the central cannulation cohort are inserted into a constantly moving organ as opposed to the more static femoral vessels. Also, unique challenges to master ECMO cannulation on neonates makes them susceptible to several detrimental events (36-38,40). As already known neonates have higher risk of myocardial stunning, systemic hypertension, hyperbilirubinemia, pulmonary hemorrhage, hemolysis, seizures and cerebrovascular events on ECMO (3,21,34). In their work Delaplain et al. showed that the low birth weight under 2 kg was not negatively predictive of survival on ECMO. However, it should be kept in mind that a large number of neonates had CDH as a primary diagnosis without cardiac involvement. Only 7% of these patients had concomitant CHD and the presence of these defects were associated with 49% increased odds of death (44). Therefore, careful patient selection and minimizing ECMO complications might help ameliorate survival in this population.
Analysis of large eCPR patient cohort by Thiagarajan et al. proposes renal dysfunction, pulmonary hemorrhage, CNS injury, cCPR during ECMO, and pre ECMO arterial blood pH <7.2 as independent risk factors for early mortality (32). In our study femoral ECMO was associated with low odds of survival, whereas it has to be taken into account that majority of these patients were in the higher-risk eCPR group. Barrett et al. described in a US-based large study cohort the hazardous effect of pulmonary hemorrhage and postoperative bleeding in patients while on ECMO support (21). In our study, both patients who suffered from severe pulmonary hemorrhage were also among our inpatient non-survivors. Repeated ECMO implantations in same patients was shown to be a signal for poor outcome as exhibited in several research articles (27,45). In the study by Sherwin et al. the analysis of ELSO registry data identified 28 patients with more than one ECMO implantation during the same admission revealing only a single survivor (39). Similarly, our study showed an extremely poor outcome with no survivors after repeated ECMO implantation.
Fluid overload is common in ECMO patients and can be managed with diuretics or hemodiafiltration/peritoneal dialysis and determines one of the main indications for renal replacement therapy. In order to decrease pulmonary interstitial edema negative fluid balance was maintained whenever it was possible in our study. Although it did not yield statistical significance, in this study renal replacement therapy was associated with high rates of mortality on ECMO (7 of 11 patients treated with CVVH and 8 of 15 patients who undergone peritoneal dialysis were among in-hospital non-survivors). This finding is consistent with increased disease severity and higher rate of multiorgan failure as described in several previous studies (16,19,27,29).
In the present study the only patient with accidental hypothermia after cold water drowning died despite ECMO support. Given the fact that it was one single patient, it is impossible to generalize it regarding the suitability of ECMO to this indication category. A study by Saczkowski et al. analyzing larger patient cohort showed very promising outcomes of ECMO therapy for this patient category (46).
Surprisingly, malignant arrhythmias did not affect ECMO survival in our cohort, whereas the study by Saczkowski et al. along with several other studies identified rhythm disturbances as factors negatively impacting outcome. Ford et al. studied a large number of ECMO cases in neonates with cardiac disease and showed sepsis and renal failure to be negative predictors for ECMO survival (28). However, our results did not reach statistical significance, these complications were associated with high rates of mortality.
The incidence of cerebrovascular events on ECMO was shown to be 5–13% as stated by ELSO registry report (22). The neonates are on higher risk of IVH and white matter injury due to poor cerebral autoregulation (39,42). Pre ECMO and post ECMO neurologic impairments were also common causes of death in children in the present study. In a single-center study performed in Toronto as well as several other studies neurological complications were also a major cause of mortality and morbidity, whereas electrographic only seizures and status epilepticus may denote the underlying brain injury and have significant negative impact on survival as well (16,27,28).
Only a very small number of vvECMO patients in this study represent a limitation in terms of extrapolating results related to this small patient cohort. In brief, one girl died as a result of ECMO cannula malposition and one boy with a long medical history of complex airway obstruction did not survive owing to the rare innate anatomic pathology.
As in present study cohort publication by Ford et al. showed a statistically higher pre ECMO positive airway pressure in non-survivors (28). This relationship may reflect the irreversible damage of lung function leading to death in patients depending on high respiratory support for sufficient oxygenation.
Another interesting point that was found in our study is the low ionized calcium (Ca2+) level in non-survivors. Whereas there is lack of literature on this particular topic, it can be speculated that the low ionized Ca level in non-survivors in our patient population might be linked to the frequent use of crystalloid priming which creates dilutional hypocalcemia.
Corroborating our findings working group by von Bahr et al. revealed excellent long-term survival rates with plateaued Kaplan-Meier curves in patients who survived first month after ECMO treatment (47). Whereas survival is of fundamental importance, quality of life should also be appropriately considered and accounted for. Therefore, it is crucial for physicians to be judicious in patient selection for ECMO treatment and to discuss realistic expectations with families and next of kin. Generally, average intelligence of neonatal and pediatric ECMO survivors may not be lower than of their peers, however long-term neuropsychological compromise and school problems are prevalent (11). At school ages difficulties with spatial and processing tasks (up to 26%), reading comprehension (up to 39%), and behavior disorders are common. Also, adult survivors evaluated in late adolescence experience verbal, visual-spatial, and working memory problems (48-50). Many of children live and thrive with complex medical problems and the quality of life differs from the control population. These facts emphasize that life quality of ECMO survivors represents an important area of concern for patients and as well as for their parents and primary caregivers. On the other hand, paucity of literature on this topic implies the importance of large, prospective, multicenter studies for better understanding of the functional status in long-term pediatric and neonatal ECMO survivors.
Study limitations
This study is limited by its retrospective nature, heterogeneity of patient population and relatively small number of patients from a single center. Also, the retrospective nature of this study may introduce confounders including selection, information and misclassification bias. The missing values for many variables precluded their inclusion in statistical analysis.
Conclusions
ECMO provides efficient therapy opportunities for life-threatening conditions. Nevertheless, neonates and pediatric patients who underwent ECMO/ECLS are at high risk for cerebrovascular events and poor ECMO survival. Moreover, prolonged duration on ECMO support might aggravate irreversible cardiopulmonary failure. Appropriate patient selection using predictors of outcome and efforts to reduce complications might improve outcomes of this demanding patient cohort.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The institutional review board of Cologne University approved this study and waived the need for individual patient consent. All procedures followed were in accordance with ethical standards, current guidelines and Helsinki Declaration.
References
- Wolfson PJ. The development and use of extracorporeal membrane oxygenation in neonates. Ann Thorac Surg 2003;76:S2224-9. [Crossref] [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- International Summary of ELSO Report. Extracorporeal Life Support Organization (ELSO). Ann Arbor, MI, January and July 2017.
- American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2017. Available online: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/
- Gournay V, Hauet Q. Mechanical circulatory support for infants and small children. Arch Cardiovasc Dis 2014;107:398-405. [Crossref] [PubMed]
- Chauhan S, Malik M, Malik V, et al. Extra corporeal membrane oxygenation after pediatric cardiac surgery: A 10 year experience. Ann Card Anaesth 2011;14:19-24. [PubMed]
- Prodhan P, Fiser RT, Dyamenahalli U, et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the Intensive Care Unit. Resuscitation 2009;80:1124-9. [Crossref] [PubMed]
- Kachel W, Arnold D, Rettwitz W, et al. Extracorporeal membrane oxygenation (ECMO). A treatment alternative for newborn infants with severe respiratory disorder. Monatsschr Kinderheilkd 1987;135:735-41. [PubMed]
- Lin JC. Extracorporeal Membrane Oxygenation for Severe Pediatric Respiratory Failure. Respir Care 2017;62:732-50. [Crossref] [PubMed]
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Yu YR, Carpenter JL, DeMello AS, et al. Evaluating quality of life of extracorporeal membrane oxygenation survivors using pediatric quality of life inventory survey. J Pediatr Surg 2018;53:1060-4. [Crossref] [PubMed]
- Ham PB, Hwang B, Wise LJ, et al. Venovenous extracorporeal membrane oxygenation in Pediatric Respiratory Failure. Am Surg 2016;82:787-8. [PubMed]
- Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: History, development and current status. World J Crit Care Med 2013;2:29-39. [Crossref] [PubMed]
- Mahmood B, Newton D, Pallotto EK. Current trends in neonatal ECMO. Semin Perinatol 2018;42:80-8. [Crossref] [PubMed]
- Ford JW. Neonatal ECMO: current controversies and trends. Neonatal Netw 2006;25:229-38. [Crossref] [PubMed]
- Balasubramanian SK, Tiruvoipati R, Amin M, et al. Factors influencing the outcome of paediatric cardiac surgical patients during extracorporeal circulatory support. J Cardiothorac Surg 2007;2:4. [Crossref] [PubMed]
- Aiello S, Loomba RS. Factors Associated with the Need for, and the Impact of, Extracorporeal Membrane Oxygenation in Children with Congenital Heart Disease during Admissions for Cardiac Surgery. Children (Basel) 2017;4. [Crossref] [PubMed]
- Baslaim G, Bashore J, Al-Malki F, et al. Can the outcome of pediatric extracorporeal membrane oxygenation after cardiac surgery be predicted? Ann Thorac Cardiovasc Surg 2006;12:21-7. [PubMed]
- Kumar TK, Zurakowski D, Dalton H, et al. Extracorporeal membrane oxygenation in postcardiotomy patients: Factors influencing outcome. J Thorac Cardiovasc Surg 2010;140:330-336.e2. [Crossref] [PubMed]
- Chan T, Barrett CS, Tjoeng YL, et al. Racial variations in extracorporeal membrane oxygenation use following congenital heart surgery. J Thorac Cardiovasc Surg 2018;156:306-15. [Crossref] [PubMed]
- Barrett CS, Chan T, Wilkes J, et al. Association of Pediatric Cardiac Surgical Volume and Mortality after Cardiac ECMO. ASAIO J 2017;63:802-9. [Crossref] [PubMed]
- ECLS Registry Report 2017. ELSO Extracorporeal life support organisation.
- Aharon AS, Drinkwater DC Jr, Churchwell KB, et al. Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. Ann Thorac Surg 2001;72:2095-101. [Crossref] [PubMed]
- Faraoni D, Nasr VG, DiNardo JA, et al. Hospital costs for neonates and children supported with extracorporeal membrane oxygenation. J Pediatr 2016;169:69-75.e1. [Crossref] [PubMed]
- Johnston L, Williams SB, Ades A. Education for ECMO providers: Using education science to bridge the gap between clinical and educational expertise. Semin Perinatol 2018;42:138-46. [Crossref] [PubMed]
- Marino BS, Tabbutt S, MacLaren G, et al. Cardiopulmonary Resuscitation in Infants and Children With Cardiac Disease: A Scientific Statement From the American Heart Association. Circulation 2018;137:e691-e782. [Crossref] [PubMed]
- Alsoufi B, Al-Radi OO, Gruenwald C, et al. Extra-corporeal life support following cardiac surgery in children: analysis of risk factors and survival in a single institution. Eur J Cardiothorac Surg 2009;35:1004-11. [Crossref] [PubMed]
- Ford MA, Gauvreau K, McMullan DM, et al. Factors Associated With Mortality in Neonates Requiring Extracorporeal Membrane Oxygenation for Cardiac Indications: Analysis of the Extracorporeal Life Support Organization Registry Data. Pediatr Crit Care Med 2016;17:860-70. [Crossref] [PubMed]
- Kane DA, Thiagarajan RR, Wypij D, et al. Rapid-response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation 2010;122:S241-8. [Crossref] [PubMed]
- Furlong-Dillard JM, Amula V, Bailly DK, et al. Use of Extracorporeal Membrane Oxygenation and Mortality in Pediatric Cardiac Surgery Patients with Genetic Conditions: A Multicenter Analysis. Pediatr Crit Care Med 2017;18:850-8. [Crossref] [PubMed]
- Masterson S, McNally B, Cullinan J, et al. Out-of-hospital cardiac arrest survival in international airports. Resuscitation 2018;127:58-62. [Crossref] [PubMed]
- Thiagarajan RR, Laussen PC, Rycus PT, et al. Extracorporeal Membrane Oxygenation to Aid Cardiopulmonary Resuscitation in Infants and Children. Circulation 2007;116:1693-700. [Crossref] [PubMed]
- Erek E, Aydın S, Suzan D, et al. Extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest in children after cardiac surgery. Anatol J Cardiol 2017;17:328-33. [PubMed]
- Bokman CL, Tashiro J, Perez EA, et al. Determinants of survival and resource utilization for pediatric extracorporeal membrane oxygenation in the United States 1997–2009. J Pediatr Surg 2015;50:809-14. [Crossref] [PubMed]
- Friedland-Little JM, Aiyagari R, Yu S, et al. Survival through staged palliation: fate of infants supported by extracorporeal membrane oxygenation after the Norwood operation. Ann Thorac Surg 2014;97:659-65. [Crossref] [PubMed]
- Oyetunji TA, Thomas A, Moon TD, et al. The impact of ethnic population dynamics on neonatal ECMO outcomes: a single urban institutional study. J Surg Res 2013;181:199-203. [Crossref] [PubMed]
- Qureshi FG, Jackson HT, Brown J, et al. The changing population of the United States and use of extracorporeal membrane oxygenation. J Surg Res 2013;184:572-6. [Crossref] [PubMed]
- Mattke CA, Haisz E, Pandya N, et al. Creating a Controlled Arterio-Venous Shunt by Reversing the Extracorporeal Membrane Oxygenation Blood Flow: A Strategy for Weaning Patients Off Veno-Arterial Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2017;18:973-6. [Crossref] [PubMed]
- Sherwin ED, Gauvreau K, Scheurer MA, et al. Extracorporeal membrane oxygenation after stage 1 palliation for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2012;144:1337-43. [Crossref] [PubMed]
- Mahle WT, Hu C, Trachtenberg F, et al. Heart failure after the Norwood procedure: An analysis of the Single Ventricle Reconstruction Trial. J Heart Lung Transplant 2018;37:879-85. [Crossref] [PubMed]
- Ungerleider RM, Shen I, Yeh T, et al. Routine mechanical ventricular assist following the Norwood procedure–improved neurologic outcome and excellent hospital survival. Ann Thorac Surg 2004;77:18-22. [Crossref] [PubMed]
- Mascio CE, Austin EH, Jacobs JP, et al. Perioperative mechanical circulatory support in children: An analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg 2014;147:658-64. [Crossref] [PubMed]
- Chrysostomou C, Morell VO, Kuch BA, et al. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg 2013;146:317-25. [Crossref] [PubMed]
- Delaplain PT, Zhang L, Chen Y, et al. Cannulating the contraindicated: effect of low birth weight on mortality in neonates with congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg 2017;52:2018-25. [Crossref] [PubMed]
- Soquet J, Chiletti R, Horton S, et al. Dismal Outcomes of Second-Run Extracorporeal Life Support in the Paediatric Population. Heart Lung Circ 2019;28:450-4. [Crossref] [PubMed]
- Saczkowski RS, Brown DJA, Abu-Laban RB, et al. Prediction of Survival in Accidental Hypothermia Requiring Extracorporeal Life Support: An Individual Patient Data Meta-Analysis. Resuscitation 2018;127:51-7. [Crossref] [PubMed]
- von Bahr V, Hultman J, Eksborg S, et al. Long-Term Survival and Causes of Late Death in Children Treated With Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med 2017;18:272-80. [Crossref] [PubMed]
- Madderom MJ, Reuser JJ, Utens EM, et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: a nationwide multicenter study. Intensive Care Med 2013;39:1584-93. [Crossref] [PubMed]
- Madderom MJ, Schiller RM, Gischler SJ, et al. Growing up after critical illness: verbal, visual-spatial, and working memory problems in neonatal extracorporeal membrane oxygenation survivors. Crit Care Med 2016;44:1182-90. [Crossref] [PubMed]
- Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Ment Retard Dev Disabil Res Rev 2002;8:234-40. [Crossref] [PubMed]


