Preservation solutions for cardiac and pulmonary donor grafts: a review of the current literature
Introduction
Despite many of the advances within the realm of transplantation, graft survival remains imperfect. Optimal preservation of the graft is an important determinant of graft survival and patient outcomes. Considerable attention is given to the ex vivo period as this segment represents a vulnerable timeframe whereby organs are susceptible to ongoing cellular damage that is further compounded by reperfusion injury upon re-anastomosis. Hypothermia is utilized to decrease the metabolic activity of donor organs during the ex vivo period. Decrease donor organ temperature from 37 to 4 °C results in a 12 fold decrease in the metabolic demand (1). However, hypothermia alone is unable to abolish all cellular damage as metabolism persists at approximately 5-10% of normal. In addition, hypothermia can lead to Na+/K+ ATPase alterations, ATP depletion, dysregulation of Ca2+ homeostasis, mitochondrial perturbations, xanthine oxidase accumulation, and increased levels of reactive oxygen species (ROS) which may have deleterious effects on cellular viability (2). Therefore, preservation solutions have been implemented in conjunction with hypothermia for additional cellular protection. Numerous solutions are commercially available while others remain institutionally derived.
There is continued uncertainty among clinicians regarding the most optimal preservation solution as evidenced by Demmy et al. who revealed the use of 167 different solutions among United Network for Organ Sharing (UNOS) cardiac transplant centers (3). It is clear that investigation concerning the optimal preservation solution is necessary to reduce such widespread variability and potentially improve graft outcomes. As such, we sought to review the pertinent clinical studies available in an attempt to identify characteristics of an ideal preservation solution for both cardiac and pulmonary grafts with the intention of ultimately minimizing graft dysfunction and improving patient outcomes.
Classification of preservation solutions
Preservation solutions
Euro Collins (EC) solution was designed in the 1960s and considered the preservation solution of choice for over 15 years until organ perseveration was revolutionized by the introduction of University of Wisconsin (UW) solution in 1988 (4). However, the high molecular weight compounds within UW such as hydroxyethyl starch (HES) resulted in a highly viscous solution that was implicated in part, to organ dysfunction thereby, supporting the development of less vicious alternatives including Celsior (CEL) and histidine-tryptophan-ketoglutarate (HTK) (5).
Many targeted approaches to cardiac organ preservation have been attempted including Plegisol which arose from the initial St. Thomas solution used for cardioplegia, albeit with slight modifications including the addition of a buffering system (6). In contrast to the aforementioned acellular approaches, Papworth solution was centered on the inclusion of donor blood in its composition (7). The different metabolic demand and physiology of the lung supported the construction of pulmonary specific solutions including Perfadex (PER) which still remains confined for sole use in pulmonary transplantations by the Federal Drug Administration (FDA) in the United States.
Preservation solutions are composed of multiple elements, each with their own advantages and disadvantages (Table 1). We will highlight a common classification scheme for EC, UW, HTK, CEL, PER, Papworth, and Plegisol according to respective molecular properties.
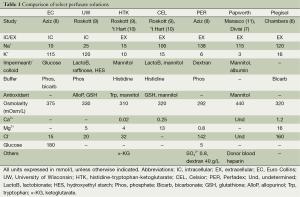
Full table
Intracellular/extracellular
Preservation solutions can be broadly classified into intracellular and extracellular solutions based upon the potassium and sodium concentrations. Intracellular solutions closely recapitulate the high potassium/low sodium conditions present within the cellular milieu to minimize potential concentration gradients across the plasma membrane that could favor potassium efflux. UW and EC are popular intracellular solutions, however the perceived risk of hyperkalemia induced pulmonary vasoconstriction favored the design of extracellular (low potassium) solutions such as HTK, CEL, PER, Papworth, and Plegisol (10). Over time, intracellular and extracellular solutions were shown to be equivalent (10).
Impermeant/colloid
Hypothermia causes dysregulation of the Na+/K+ pumps in the cellular membrane resulting in cellular edema through sodium and water influx into the cell (12). The addition of an impermeant or colloid creates an osmotic force that preferentially promotes water retention in the extracellular compartment to counteract this effect. EC contains a high concentration of glucose that was intended to act as impermeable barrier. However, glucose is suboptimal as enzymatic cleavage occurs resulting in substrate diffusion into the cell and subsequent cellular edema (2). The development of newer solutions containing alternate impermeants/colloids led to superior protection against cellular swelling. UW contains lactobionate and the trisaccharide impermeant raffinose as well as the synthetic colloid HES (Roskott et al.). HTK, CEL, and Papworth rely on mannitol to combat tissue edema (9). In addition to mannitol, lactobionate and albumin are included in CEL and Papworth, respectively for further protection (9,11).
Buffer
Many of the commercial preservation solutions contain a buffer to combat the effects of metabolic acidosis that result from the shift of aerobic to anaerobic metabolism during periods of ischemia. UW, PER, and EC utilize phosphate buffers whereas, HTK and CEL are comprised of histidine buffering systems to prevent cellular damage (8,9). Bicarbonate is an effective buffer and used in EC and Plegisol (6,8).
Antioxidants
ROS are an inevitable consequence of tissue ischemia during the ex vivo period and can lead to significant cellular damage. UW counteracts ROS with a combination of allopurinol to inhibit the formation xanthine oxidase and glutathione which can act as a reducing agent (9). Glutathione is also the mainstay of antioxidant activity in CEL (9). HTK’s antioxidant properties are attributed to tryptophan which is a functional electron donor (10). Moreover, mannitol has been suggested to have antioxidant properties which may confer a benefit to CEL, HTK, and Papworth (10).
Heart transplantation
Although ischemia times as long as 13 hours have been reported for heart transplants, cold ischemia times are usually limited to less than 6 hours (13,14). CEL was initially a favorable extracellular preservation solution for heart transplants with several studies supporting its use (Table 2). A prospective study containing 70 patients revealed a safe role for CEL as a preservation solution in the setting of heart transplants with a 30-day survival of 91.4% and acute graft failure rate of 10% (15). This was supported by De Santo et al. who found an in-hospital mortality rate of 8% and 1 year mortality rate of 12% in 200 patients that received CEL (16). Interestingly, upon stratification into low and high risk grafts in that study, there was no difference in mortality or graft failure suggesting a potential safe role for the use of CEL even in the setting of prolonged ischemia (>180 minutes) (16).
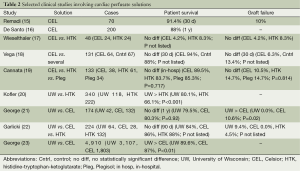
Full table
Given the suggested beneficial role of CEL, many comparison trials were performed. An evaluation of 48 patients (24 HTK and CEL 24) suggested a beneficial role for CEL as only one case of graft failure was observed in the CEL arm compared to two in the HTK group. However, the results of this study were preliminary and the low number of patients made it difficult to derive any meaningful conclusions (17). Vega et al. (18) evaluated 131 patients with the use of CEL (n=64) to several other solutions (n=67) including: UW, Plegisol, Stanford solution, PlasmaLyte A, Carmichael solution, Roe, lactate ringers, and normal saline. There was no difference in the mortality rate at 30 days (CEL 94% vs. others 88%) or graft failure rate at 30 days (CEL 6.3, Cntrl 13.4%; P not listed) (18). Although comparisons of CEL to the use of a specific solution could not be made given the variety of controls in this study, it did once again demonstrate a safe use for CEL in heart transplants. To compare CEL against a limited number of control preservation solutions, Cannata et al. (19) evaluated 133 patients (CEL 38, HTK 61, and Plegisol 34) and found no statistical difference with respect to in-hospital mortality [CEL 10.5%, HTK 16.3%, and Plegisol (St. Thomas) 14.7%, P=0.717] or graft failure (HTK 14.7%, CEL 10.5%, and Plegisol 14.7%, P=0.814).
UW emerged as a popular alternative for heart transplants as there was a survival benefit associated with its use compared to other solutions such as HTK. Kofler et al. (20) saw an improvement in survival after switching from HTK to the use of UW in their heart transplant series (UW 80.1% vs. HTK 66.1% survival at 1 year, P<0.001). During the transition to UW, that institution also began using nitric oxide and prostanoids to prevent right heart failure which may have imposed confounding effects. An evaluation of 174 patients (42 UW and 132 CEL) found no difference in 30-day/1 year mortality and primary graft dysfunction (UW 11.9% vs. CEL 26.5% P=0.059) with the use of UW (21). However, a higher rate of right heart failure was found in the CEL group (UW 0% vs. CEL 10.6% P=0.02) (21). Conflicting results were found in an evaluation of 224 patients (UW 64, HTK 132, and CEL 28) where a trend towards lower mortality at 90 days with the use of HTK was observed (UW 16%, HTK 12%, and CEL 14%) (22). Acute graft failure did not occur in the CEL group and was moderate in the UW and HTK groups (UW 9.4%, HTK 4.5%, CEL 0%; P not listed) (22).
The largest study to date was performed by George et al. (23) which addressed the mixed results observed between UW and CEL. It comprised 4,910 patients (UW 3,107 and CEL 1,803) and revealed an improvement in 1 year survival with the use of UW (UW 89.6% vs. CEL 87.0% P<0.01) (23). Graft survival was not stated (23). Although the improvement in survival is modest, it may account for the lack of statistically significant differences observed by George et al. (21) and Garlicki et al. (22) as these studies had relatively lower numbers of patients. Together these results suggest that UW should be the preservation solution of choice in heart transplants.
Lung transplantation
The lung can only tolerate a short period of ischemia, usually less than 6 hours (24). Tierney et al. (12) reported their experience with lung transplants over a one year duration using EC and prostaglandin E1 with a one year survival of 79%. Oto et al. (25) showed no difference in 30-day mortality in 157 lung transplants with the use of EC, Papworth, or PER. However, a follow up study at the same institution with a greater number of patients showed an increased correlation with long-term death associated with the use Papworth compared to EC or PER in 310 patients [216 double lung transplantations (DLT) and 94 single lung transplantations (SLT)] (11). The effect on mortality is not apparent until after 3 years, potentially accounting for the lack of difference observed among the three perfusate solutions in the Oto’s study (25). In both studies there was a lower incidence of primary graft dysfunction observed with PER (11,25). In a larger study comparing multiple solutions, Ganesh et al. (26) found no difference in risk adjusted mortality among 681 patients who received EC (284 patients), blood albumin [139], low potassium dextran (LPD) solution (commercially sold as PER), or core cooling (107 patients).
Intracellular preservation solutions were initially used in lung transplants. Hardesty et al. (27) compared the use of EC (30 patients) to UW (70 patients) in 100 transplants [13 heart-lung (HLT), 45 DLT, 42 SLT transplants). Both solutions were found to be comparable (27). Given the potential for pulmonary dysfunction from potassium induced vasoconstriction with intra-cellular solutions, extracellular preservation solutions became a topic of interest (28). Thabut et al. (29) evaluated 170 patients (124 SLT and 46 DLT) who received UW, EC, Cambridge, or CEL (n=24, 61, 64, and 21 patients, respectively). There was no difference in 1 month mortality, however there was a lower incidence of post-transplant graft edema with the use of Cambridge solution (an extracellular solution) after adjustment for the duration of graft ischemia (29). One of the largest comparison studies involving the use of UW in lung transplants was performed by Arnaoutakis et al. (30) who evaluated 4,455 patients (4,161 LPD vs. 294 UW) and found an increased risk of mortality at one year with the use of UW (hazard ratio 1.75, P=0.004) after multivariate analysis.
EC has been directly compared to PER (a LPD) in multiple studies (Table 3). Aziz et al. (8) compared the use of EC and PER in 69 patients (EC 37 and PER 32). There were 12 SLT (EC 7, PER 5), 51 DLT (EC 27, PER 24), and 6 HLT (3 EC, PER 3) (8). There was no difference in the 30-day mortality (EC 10.8% vs. PER 9.3%, P=0.88), PaO2/FiO2 ratio (EC 244 vs. PER 266 mmHg, P=0.9), or duration of mechanical ventilation (EC 71.2 vs. PER 91.9 hr, P=0.4) (8). Similar results were observed by Gámez et al. (31) who compared the use of EC to PER in 136 lung transplants [SLT (EC 32, PER 15) and DLT (EC 36, PER 53)] and found no difference in 30-day mortality, length of time on the mechanical ventilator, and PaO2/FiO2 ratio (P values not listed). However, the EC group had a higher incidence (EC 37% vs. PER 16%, P=0.01) of severe graft failure (PaO2/FiO2 <150 mmHg) despite a higher number of double lung transplant recipients in the PER group (31).
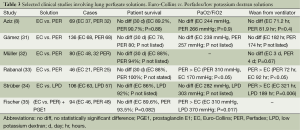
Full table
These results have been refuted by several other studies that have suggested differences between EC and PER. Müller et al. (32) evaluated 80 patients who received either EC or PER [46 SLT (EC 31 and PER 15) and 34 DLT (EC 17 and PER 17)]. There was a trend towards improved 30-day mortality (EC 12% vs. PER 6%, P not listed) and 1 year mortality (EC 62% vs. PER 79%, P not listed) associated with the use of PER (32). PER was also associated with a favorable reperfusion injury score and improved alveolar/arterial oxygen ratio while the duration of mechanical ventilation was not statistically significant (P=0.67) (32). Rabanal et al. (33) evaluated 46 patients undergoing lung transplantation who received EC or PER (EC 21, PER 25 patients). There was no statistical difference in the 30 day mortality between both groups (EC 12% and 0% PER, P not stated), however, there was a better PaO2/FiO2 ratio (EC 170 vs. PER 310, P<0.05) and lower duration of mechanical ventilation (EC 92 EC vs. PER 72, P<0.05) associated with the use of PER (33). In similar comparisons, Fischer et al. (35) also observed a lower PaO2/FiO2 (EC 310, LPD 370 mmHg; P=0.017) with the use of PER while Strüber et al. (34) observed a shorter duration of mechanical ventilation (EC 321 vs. LPD 189 hr, P=0.006) that correlated with the use of a LPD solution such as PER. Of note, the duration of mechanical ventilation in the Strüber (34) study was substantially longer than other studies such as Rabanal et al. (33).
Together these studies suggest against the use of Papworth and UW as they may impose an increased risk of mortality. In comparing two of the most commonly used extracellular preservation solutions in lung transplantation (EC and PER) there does not appear a survival benefit afforded with the use of either solution. However, the improved PaO2/FiO2 and lower duration of mechanical ventilation observed in some studies favor the use of PER.
Conclusions
Based upon the aforementioned studies, UW is superior for cardiac transplantation with a slight survival advantage compared to CEL while PER is the preferred solution for pulmonary transplantations. The use of PER correlates with an improved PaO2/FiO2 ratio and a shorter duration of mechanical ventilation. While we looked at graft survival and overall patient survival, it should be noted that these outcomes are not solely dependent on the preservation solution used. Several variables such as the quality of the graft, surgical technique, and immunosuppression regimen have important contributions to the overall success. Additionally, the survival time point used in our review may not have encompassed the long-term effects associated with the use of a particular preservation solution. Many of the studies were also limited by small sample sizes and may have been underpowered to detect minute differences. The optimal preservation solution for each respective organ can be supported by available evidence based data and might be a useful adjunct to ameliorate the widespread viability observed by Demmy et al. (3) among different centers.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 1988;45:673-6. [PubMed]
- Guibert EE, Petrenko AY, Balaban CL, et al. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus Med Hemother 2011;38:125-42. [PubMed]
- Demmy TL, Biddle JS, Bennett LE, et al. Organ preservation solutions in heart transplantation--patterns of usage and related survival. Transplantation 1997;63:262-9. [PubMed]
- Mühlbacher F, Langer F, Mittermayer C. Preservation solutions for transplantation. Transplant Proc 1999;31:2069-70. [PubMed]
- Feng XN, Xu X, Zheng SS. Current status and perspective of liver preservation solutions. Hepatobiliary Pancreat Dis Int 2006;5:490-4. [PubMed]
- Chambers DJ, Sakai A, Braimbridge MV, et al. Clinical validation of St. Thomas’ Hospital cardioplegic solution No. 2 (Plegisol). Eur J Cardiothorac Surg 1989;3:346-52. [PubMed]
- Divisi D, Montagna P, Jegaden O, et al. A comparative study of Euro-Collins, low potassium University of Wisconsin and cold modified blood solutions in lung preservation in acute autotransplantations in the pig. Eur J Cardiothorac Surg 2001;19:333-8. [PubMed]
- Aziz TM, Pillay TM, Corris PA, et al. Perfadex for clinical lung procurement: is it an advance? Ann Thorac Surg 2003;75:990-5. [PubMed]
- Roskott AM, Nieuwenhuijs VB, Dijkstra G, et al. Small bowel preservation for intestinal transplantation: a review. Transpl Int 2011;24:107-31. [PubMed]
- ’t Hart NA, Leuvenink HGD, Ploeg RJ. New Solutions in Organ Preservation. Transplantation Rev 2002;16:131-41.
- Marasco SF, Bailey M, McGlade D, et al. Effect of donor preservation solution and survival in lung transplantation. J Heart Lung Transplant 2011;30:414-9. [PubMed]
- Tierney A, Foster R, Ogella D. A perfusionist’s role in lung transplant preservation. Perfusion 2004;19:351-7. [PubMed]
- Wei J, Chang CY, Chuang YC, et al. Successful heart transplantation after 13 hours of donor heart ischemia with the use of HTK solution: a case report. Transplant Proc 2005;37:2253-4. [PubMed]
- Southard JH, Belzer FO. Organ preservation. Annu Rev Med 1995;46:235-47. [PubMed]
- Remadi JP, Baron O, Roussel JC, et al. Myocardial preservation using Celsior solution in cardiac transplantation: early results and 5-year follow-up of a multicenter prospective study of 70 cardiac transplantations. Ann Thorac Surg 2002;73:1495-9. [PubMed]
- De Santo LS, Amarelli C, Romano G, et al. High-risk heart grafts: effective preservation with Celsior solution. Heart Vessels 2006;21:89-94. [PubMed]
- Wieselthaler GM, Chevtchik O, Konetschny R, et al. Improved graft function using a new myocardial preservation solution: Celsior. Preliminary data from a randomized prospective study. Transplant Proc 1999;31:2067-8. [PubMed]
- Vega JD, Ochsner JL, Jeevanandam V, et al. A multicenter, randomized, controlled trial of Celsior for flush and hypothermic storage of cardiac allografts. Ann Thorac Surg 2001;71:1442-7. [PubMed]
- Cannata A, Botta L, Colombo T, et al. Does the cardioplegic solution have an effect on early outcomes following heart transplantation? Eur J Cardiothorac Surg 2012;41:e48-52; discussion e52-3.
- Kofler S, Bigdeli AK, Kaczmarek I, et al. Long-term outcomes after 1000 heart transplantations in six different eras of innovation in a single center. Transpl Int 2009;22:1140-50. [PubMed]
- George TJ, Arnaoutakis GJ, Beaty CA, et al. A novel method of measuring cardiac preservation injury demonstrates University of Wisconsin solution is associated with less ischemic necrosis than Celsior in early cardiac allograft biopsy specimens. J Heart Lung Transplant 2012;31:410-8. [PubMed]
- Garlicki M, Kołcz J, Rudziński P, et al. Myocardial protection for transplantation. Transplant Proc 1999;31:2079-83. [PubMed]
- George TJ, Arnaoutakis GJ, Baumgartner WA, et al. Organ storage with University of Wisconsin solution is associated with improved outcomes after orthotopic heart transplantation. J Heart Lung Transplant 2011;30:1033-43. [PubMed]
- Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med 1999;340:1081-91. [PubMed]
- Oto T, Griffiths AP, Rosenfeldt F, et al. Early outcomes comparing Perfadex, Euro-Collins, and Papworth solutions in lung transplantation. Ann Thorac Surg 2006;82:1842-8. [PubMed]
- Ganesh JS, Rogers CA, Banner NR, et al. Does the method of lung preservation influence outcome after transplantation? An analysis of 681 consecutive procedures. J Thorac Cardiovasc Surg 2007;134:1313-21. [PubMed]
- Hardesty RL, Aeba R, Armitage JM, et al. A clinical trial of University of Wisconsin solution for pulmonary preservation. J Thorac Cardiovasc Surg 1993;105:660-6. [PubMed]
- Okada Y, Kondo T. Preservation solution for lung transplantation. Gen Thorac Cardiovasc Surg 2009;57:635-9. [PubMed]
- Thabut G, Vinatier I, Brugière O, et al. Influence of preservation solution on early graft failure in clinical lung transplantation. Am J Respir Crit Care Med 2001;164:1204-8. [PubMed]
- Arnaoutakis GJ, Allen JG, Merlo CA, et al. Low potassium dextran is superior to University of Wisconsin solution in high-risk lung transplant recipients. J Heart Lung Transplant 2010;29:1380-7. [PubMed]
- Gámez P, Córdoba M, Millán I, et al. Improvements in lung preservation: 3 years’ experience with a low-potassium dextran solution. Arch Bronconeumol 2005;41:16-9. [PubMed]
- Müller C, Fürst H, Reichenspurner H, et al. Lung procurement by low-potassium dextran and the effect on preservation injury. Munich Lung Transplant Group. Transplantation 1999;68:1139-43. [PubMed]
- Rabanal JM, Ibañez AM, Mons R, et al. Influence of preservation solution on early lung function (Euro-Collins vs Perfadex). Transplant Proc 2003;35:1938-9. [PubMed]
- Strüber M, Wilhelmi M, Harringer W, et al. Flush perfusion with low potassium dextran solution improves early graft function in clinical lung transplantation. Eur J Cardiothorac Surg 2001;19:190-4. [PubMed]
- Fischer S, Matte-Martyn A, De Perrot M, et al. Low-potassium dextran preservation solution improves lung function after human lung transplantation. J Thorac Cardiovasc Surg 2001;121:594-6. [PubMed]


