
Intraoperative near infrared fluorescence imaging for the assessment of the gastric conduit
Introduction
Esophagectomy is the primary treatment modality for some early-stage esophageal cancers, part of the armamentarium in the treatment of locally advanced esophageal cancer, and an option in certain end-stage benign esophageal conditions. Recent literature suggests that the rates of complications in esophagectomy are as high as 59% (1). Aside from iatrogenic airway injury, anastomotic leak and gastric tip necrosis represent the constellation of complications that surgeons fear most. Anastomotic leak has been shown to be an independent risk factor for mortality in this population and has been shown to decrease the oncologic value of the operation (2,3). Many factors contribute to the development of anastomotic complications including location of anastomosis, the use of radiation, conduit width, conduit used and surgeon volume (4,5). However, vascular perfusion to the anastomotic regions is believed to be the primary driver of anastomotic leak and gastric tip necrosis (6,7).
In the construction of gastric conduits, all the blood vessels to the stomach are sacrificed except the gastroepiploic arcade. This arcade runs from the gastroduodenal artery up the greater curve of the stomach and then ends approximately two-third of the way from the tip of the fundus. However, there is a submucosal vascular plexus that supplies the rest of the gastric tube as the short gastric vessels have been divided. Often, part of the conduit is removed for this reason but there are scenarios when the entire length of the conduit needs to be used despite maximal mobilization. Estimation of the vascular perfusion of this area where anastomoses are performed has largely been left to the surgeon’s objective qualification based on visual feedback. These judgments are by no means foolproof as demonstrated by the anastomotic leak rates as high as 27% reported in the literature (2).
The use of intraoperative fluorescence imaging (IFI) has gained popularity as a tool to augment surgical decision-making (8-10). In this technique, a fluorophore such as indocyanine green (ICG) is introduced into the circulation which is then subsequently delivered to the conduit and the vascular arcade which can then be visualized intraoperatively using a fluorescence detecting device. In this review, we seek to discuss the use of this method to assess quality of the neo-esophageal conduits.
ICG
ICG is a water-soluble anionic, amphiphilic infrared fluorophore with a molecular weight of 774.9 kDA. When injected, ICG binds to plasma proteins and forms a circulating nanoparticle. The main mechanism of excretion is hepatic as the liver excretes more than 80% of the available ICG in less than 24 hours (11). ICG is the most intensively studied near-infrared (NIR) contrast agent and was approved by the United States Food and Drug Administration (FDA) for human administration in 1958. As a fluorophore, ICG has a peak absorption wavelength of 805 nm and a peak emission wavelength of 830 nm. This spectrum allows for detection of signal from as deep as 10 mm within tissue (12). ICG is relatively cheap, non-toxic and readily available, making it an ideal contrast agent for IFI. The FDA specifically approves ICG for cardiac output, hepatic function and ophthalmic angiography.
Feasibility of IFI
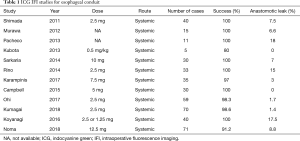
An early case series by Kubota et al. demonstrated that IFI of the neo-esophageal conduit is safe and reproducible (13). They describe a series of 5 patients who underwent esophagectomy for cancer. The study confirmed that arterial flow in the neo-esophageal conduits could be detected at doses of 0.5 mg/kg with visualization of venous flow 30 seconds later. They also report one case of a colonic conduit with apparent suboptimal perfusion by macroscopic inspection with demonstrable flow by IFI. None of these patients suffered any anastomotic complications (Table 1).
Full table
Pacheco et al. described use of IFI in 11 patients undergoing transhiatal esophagectomy (14). In these open operations, IFI identified good perfusion in 10 patients, with anastomotic leak occurring in one of these patients. An additional leak occurred in one patient with compromised perfusion identified by IFI, but not by gross clinical inspection.
Murawa et al. performed IFI in 15 patients undergoing transhiatal esophagectomy (15). IFI identified suspected vascular compromise in 4 patients. The only anastomotic leak, however, occurred in a patient thought to have adequate perfusion by IFI.
Shimada et al. utilized IFI in 40 patients undergoing cervical anastomoses (16). IFI was used to assess perfusion in multiple conduit organs including stomach, colon, and free jejunal grafts. Three anastomotic leaks occurred in patients in which IFI could not identify microcirculation. This finding, however, was not specific, given 15 of 18 patients with non-visualized microcirculation by IFI did not develop anastomotic dehiscence.
Rino et al. ran a larger study including 33 patients to assess vascular perfusion (17). The main focus of this study was to determine blood flow at the anastomotic site and whether it derived from the right gastroepiploic, left gastroepiploic or the vascular bed from the splenic hilum. Perfusion was seen in all the conduits. However, they found that 80% of the anastomotic leaks (4) occurred in patients categorized as having the splenic hilum perfusion pathway. No statistical analysis was performed but findings would indicate that relying on large anastomosis vessels through the remaining short gastric vessel arcade is sub-optimal. These studies together demonstrated early feasibility of IFI for assessing the vascular perfusion of esophagectomy conduits.
Sarkaria et al. utilized IFI in 30 patients undergoing robotic assisted minimally invasive esophagectomy to assess and clearly identify the gastric vasculature during mobilization of the greater curve (18). IFI fluorescence angiography identified termination of the gastroepiploic vascular arcade in all cases within a median 37 seconds after IV administration of 10 mg of ICG. Subjectively, IFI often identified otherwise non-visualized smaller transverse vessels between the terminal arcade and the short gastric arteries, as well as between short gastric arteries. Anecdotally in this small early series, gross intra-corporeal visualization of the conduit via IFI was not thought to significantly contribute to assessment of ischemia beyond standard white light assessment of tissue perfusion.
Optimization of location of anastomosis
As a natural step, investigators turned to using IFI to improve the location of the anastomosis to prevent anastomotic complication. Karampinis et al. performed a study where 35 patients had 7.5 mg of ICG injected with imaging performed 20 minutes later to determine the zone of optimal perfusion (19). All efforts were made to perform the anastomosis in this zone. In 33 cases, the anastomosis could be performed in this zone with one (3%) anastomotic leak. There was a marked improvement in anastomotic leaks (18.2% vs. 3.0%) when compared to internal controls of the previous 55 patients who had esophagectomy by the same technique and surgeon. When patients who were not anastomosed in the optimal zone are included in this analysis, there was no statistically significant difference in leakage (P=0.23) which would indicate that the difference in leakage is due to performing the anastomosis in the optimal zone.
Campbell et al. combined IFI and Doppler assessment of conduits to reduce anastomotic leakage (20). In conjunction with an assessment of Doppler signal strength, an area was marked on the conduit that had 75% of the perfusion of the antrum, a value that was arbitrarily set. In all 30 patients in the intervention arm, the anastomosis was able to be made at or proximal to this area with no anastomotic leakages were observed. The intervention arm was compared the outcomes of the previous 60 cases performed at the same institution. The study found a marked improvement in anastomotic leakage (20% vs. 0%, P=0.07) using the 75% rule although there was no significant difference in morbidity or 30- or 90-day mortality.
Timing of conduit perfusion and leakage
Many groups recognize that static visualization of blood vessels by IFI may not accurately characterize the perfusion of the neo-esophageal conduit and have moved to dynamic visualization of the conduits. Specifically, they have aimed to determine if there is a target time to perfusion that would better identify the optimal zone for perfusion.
Ohi et al. performed an IFI based study on 59 patients (20,21). All patients were infused with 2.5 mg of ICG and the perfusion of the conduit was inspected from 0 to 60 seconds. Regions that perfused between 15 and 40 seconds were considered rapid perfusing and those between 40 and 60 seconds were considered slow-perfusing. Thirty-two patients had anastomosis performed in rapid perfusion areas and 18 in slow-perfusion areas. Of the remaining 9 patients who would needed an anastomosis in a zero-perfusion area—the anastomotic technique was changed in three, the anastomotic route was changed in five and one patient had a vessel supercharged to a neck vessel. Overall, only 1 patient of 59 leaked and as compared to the previous 61 patient who received esophagectomy, there was a marked decrease in leakage (14.8% vs. 1.7%). Similar studies have shown that anastomosis in an area of the conduit where IFI detected ICG perfusion in less than 60 seconds was associated with a 0% leak rate as opposed to anastomosis performed in areas where perfusion was detected after 60 seconds, which was associated with a 33% leak rate (22). Of note, 30% of the conduits in the study were trimmed to allow the area of anastomosis to be in an area that was perfused in less than 60 seconds.
Koyanagi et al. describe a series of 40 patients whose perfusion of the gastroepiploic arcade and conduit wall were monitored after receiving a dose of ICG (23). They took measurements of various distances from the pylorus to these points. They found that in patients who had delayed conduit perfusion compared to simultaneous perfusion of the conduit and arcade, there was a statistically higher rate of anastomotic leaks (47% vs. 0%). They also found that an ICG flow rate of less than 1.76 cm/s2 was strongly associated with anastomotic leakage.
In the most recent and perhaps strongest evidence for this approach is a recent report from Noma (24). They compared the post-operative outcomes of 285 patients before and after initiation of an ICG IFI protocol. Essentially, the gastric conduit and area of potential anastomosis was imaged after injection of 12.5 mg of ICG. Should perfusion be visualized by 20 seconds, the anastomosis was performed in this area and if anastomotic areas were perfused within 30 seconds, further mobilization was performed prior to anastomosis creation. If perfusion was not visualized in the anastomotic area by 30 seconds, the conduit was “super charged” by the addition of a microvascular anastomosis. Post-operative outcomes of the 71 patients in this protocol was compared with the 214 previous patients using propensity-matching based on age, sex, MBI, ASA, neoadjuvant therapy, route of conduit and anastomotic type. The study found that anastomotic leak rates in patient in the IFI protocol were statistically lower than those before protocol initiation (8.8% vs. 22%, P=0.03). There was also a significant decrease in the number of intensive care unit (ICU) days by 1.1 days (P=0.02). Of note, perioperative hospital mortality was not significantly different.
Discussion
Anastomotic leakage continues to be a vexing and important challenge for thoracic surgeons to overcome. It is clear that meticulous detail to technique and quality of conduit play important roles in reducing these complications. The quality of conduit construction is largely dependent on preserving macroscopic vessels and ensuring the submucosal vascular plexus remains intact to perfuse the anastomotic site. Traditionally, experienced surgeons are successful in achieving these goals, as demonstrated in extremely low rates of anastomotic leakage (25). However, IFI as an adjunct demonstrates some value in locating sites of optimal perfusion through both static and dynamic imaging techniques. Though dynamic inspection makes more intuitive sense, the limited data available not does not indicate a clear improvement over a static inspection at a set point.
There are significant limitations on these studies as they are essentially large case-control studies. There is a lack of randomized control data that could strengthen the case for routine use of IFI. Also, the fluorophore dosage and timing protocols in these studies vary significantly. Similarly, there is little to no standardization of the surgeon, technique, or conduit used in these operations. Lastly, IFI does require the utilization of highly specialized equipment that can be expensive and difficult to acquire.
IFI has been demonstrated to have potential in many thoracic surgery applications although this technique remains in its infancy (26). For the assessment of esophageal conduits, there is a potential role to decrease anastomotic leakage and reduce the morbidity and mortality associated with the procedure. As surgical techniques for esophagectomy move more towards minimally invasive and robotic techniques, adjuncts to visual feedback will be needed as haptic feedback decreases. Thoracic surgeons must continue to decrease the risks associated with esophagectomy while continuing to provide maximal benefit.
Acknowledgements
The authors acknowledge Ms. Kathy Lovas for her editorial support.
Footnote
Conflicts of Interest: IS Sarkaria has received speaking and education honoraria from Intuitive Surgical, Inc. The other authors have no conflicts of interest to declare.
References
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004;198:42-50. [Crossref] [PubMed]
- Michelet P, D'Journo XB, Roch A, et al. Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest 2005;128:3461-6. [Crossref] [PubMed]
- Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery 2010;148:831-8; discussion 8-40. [Crossref] [PubMed]
- Pham TH, Perry KA, Enestvedt CK, et al. Decreased conduit perfusion measured by spectroscopy is associated with anastomotic complications. Ann Thorac Surg 2011;91:380-5. [Crossref] [PubMed]
- Gooszen JA, Goense L, Gisbertz SS, et al. Intrathoracic versus cervical anastomosis and predictors of anastomotic leakage after oesophagectomy for cancer. Br J Surg 2018;105:552-60. [Crossref] [PubMed]
- Okusanya OT, DeJesus EM, Jiang JX, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg 2015;150:28-35.e1. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
- Keating JJ, Kennedy GT, Singhal S. Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2015;149:e51-3. [Crossref] [PubMed]
- Moody ED, Viskari PJ, Colyer CL. Non-covalent labeling of human serum albumin with indocyanine green: a study by capillary electrophoresis with diode laser-induced fluorescence detection. J Chromatogr B Biomed Sci Appl 1999;729:55-64. [Crossref] [PubMed]
- De Grand AM, Lomnes SJ, Lee DS, et al. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J Biomed Opt 2006;11:014007. [Crossref] [PubMed]
- Kubota K, Yoshida M, Kuroda J, et al. Application of the HyperEye Medical System for esophageal cancer surgery: a preliminary report. Surg Today 2013;43:215-20. [Crossref] [PubMed]
- Pacheco PE, Hill SM, Henriques SM, et al. The novel use of intraoperative laser-induced fluorescence of indocyanine green tissue angiography for evaluation of the gastric conduit in esophageal reconstructive surgery. Am J Surg 2013;205:349-52; discussion 352-3. [Crossref] [PubMed]
- Murawa D, Hunerbein M, Spychala A, et al. Indocyanine green angiography for evaluation of gastric conduit perfusion during esophagectomy--first experience. Acta Chir Belg 2012;112:275-80. [Crossref] [PubMed]
- Shimada Y, Okumura T, Nagata T, et al. Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus 2011;8:259-66. [Crossref] [PubMed]
- Rino Y, Yukawa N, Sato T, et al. Visualization of blood supply route to the reconstructed stomach by indocyanine green fluorescence imaging during esophagectomy. BMC Med Imaging 2014;14:18. [Crossref] [PubMed]
- Sarkaria IS, Bains MS, Finley DJ, et al. Intraoperative near-infrared fluorescence imaging as an adjunct to robotic-assisted minimally invasive esophagectomy. Innovations (Phila) 2014;9:391-3. [PubMed]
- Karampinis I, Ronellenfitsch U, Mertens C, et al. Indocyanine green tissue angiography affects anastomotic leakage after esophagectomy. A retrospective, case-control study. Int J Surg 2017;48:210-4. [Crossref] [PubMed]
- Campbell C, Reames MK, Robinson M, et al. Conduit Vascular Evaluation is Associated with Reduction in Anastomotic Leak After Esophagectomy. J Gastrointest Surg 2015;19:806-12. [Crossref] [PubMed]
- Ohi M, Toiyama Y, Mohri Y, et al. Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus 2017;14:351-9. [Crossref] [PubMed]
- Kumagai Y, Hatano S, Sobajima J, et al. Indocyanine green fluorescence angiography of the reconstructed gastric tube during esophagectomy: efficacy of the 90-second rule. Dis Esophagus 2018.31. [PubMed]
- Koyanagi K, Ozawa S, Oguma J, et al. Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: New predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore) 2016;95:e4386. [Crossref] [PubMed]
- Noma K, Shirakawa Y, Kanaya N, et al. Visualized Evaluation of Blood Flow to the Gastric Conduit and Complications in Esophageal Reconstruction. J Am Coll Surg 2018;226:241-51. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Okusanya OT, Hess NR, Luketich JD, et al. Infrared intraoperative fluorescence imaging using indocyanine green in thoracic surgery. Eur J Cardiothorac Surg 2018;53:512-8. [Crossref] [PubMed]


