Video-assisted mediastinoscopic resection compared with video-assisted thoracoscopic surgery in patients with esophageal cancer
Introduction
Esophageal cancer is a malignancy that poses a serious threat to human health, with a mortality of up to over 150,000 people each year, accounting for the vast majority of the world’s deaths of esophageal cancer (1,2). Surgery is currently the preferred option with which cure of early esophageal cancer can be expected (3-5). The introduction of endoscopic-assisted minimally invasive esophageal surgery provides a new way with reduced damage to chest associated with invasive thoracic operation, decreased surgical mortality and improved postoperative quality of life for patients (6). As mediastinoscopy has altered the traditional surgical approach, chest operation is avoided, thus maintaining the integrity of the pleural cavity with less injury (7). This technique is therefore carried out in a large number of medical centers (7-9). Currently, the indications and contraindications for video-assisted mediastinal endoscopic resection of esophageal cancer have remained controversial and any widely accepted standards are yet to come (10). The purposes of this study were to compare the advantages and disadvantages of video-assisted mediastinoscopic and thoracoscopic esophageal surgery, and to provide a preliminary summary of the indications and contraindications for the mediastinoscopic esophageal resection.
Patients and methods
Patients
The data of 109 patients with T1 esophageal cancer who underwent video-assisted mediastinoscopic resection (VAMS group) in Third Affiliated Hospital of Soochow University Hospital from December 2005 to December 2011 were collected in the study for comparison with the 58 T1 esophageal cancer patients who underwent video-assisted thoracoscopic surgery (VATS group) in Zhongshan Hospital, Fudan University. In total, there were 60 men and 49 women aged 54 to 78 years (with a median age of 62 years) in the VAMS group; and 32 men and 26 women aged 55-72 years (with a median age of 62 years) in the VATS group. There was no significant difference in terms of age, sex and other demographic parameters between the two groups.
The same inclusion criteria were applied for both groups: diagnosis of esophageal squamous cell carcinoma by endoscopy; no obvious lymph node enlargement on preoperative CT examination of the chest and upper abdominal ultrasound; and no obvious enlargement of mediastinal lymph nodes on preoperative EUS, and esophageal tumor infiltration not exceeding than T1. Exclusion criteria were as the following: with a previous history of malignancy; obvious lymph node enlargement on preoperative CT examination of the chest and upper abdominal ultrasound; obvious enlargement of mediastinal lymph nodes on preoperative EUS, and esophageal tumor infiltration exceeding than T1.
Pre-operative preparation
All patients underwent an upper gastrointestinal series, enhanced chest CT and EUS for confirmation of early esophageal cancer without significantly enlarged mediastinal lymph nodes. The preoperative staging was T1-2N0M0. Routine preoperative examination was conducted to rule out any obvious contraindication for surgery.
Surgical techniques and postoperative treatment
Two concurrent operations were performed for patients in VAMS group. For the neck surgery, an incision was made along the left anterior sternocleidomastoid edge (up to the midpoint of the sternocleidomastoid and down to the jugular notch, about 5 cm long). The upper and lower esophageal segments were separated respectively through video-assisted mediastinoscopy and the diaphragm hiatus. To avoid damage to the recurrent laryngeal nerve, the separation went down along the left posterior region of the esophagus with an attempt to divide the vagus nerve, followed by the upper esophageal segment, to prevent pulling of the recurrent laryngeal nerve. The nutritional support branch from the aorta for the esophagus was clipped using a titanium clip down to the level of the pulmonary veins. Paraesophageal mediastinal lymph node dissection was carried out during this process. In the case of ruptured pleural cavity or the need of thoracic lymph node dissection, closed thoracic drainage was used to drain the pleural fluid. A silicone ball was placed for drainage after surgery, which was removed after 2-3 days as soon as the mediastinal drainage was significantly reduced.
The patients were placed in a lateral position in the VATS group. The four-port technique was then used, with a 10-mm incision in the 7th intercostal space at the midaxillary line for thoracoscope placement, a 5-mm and a 10-mm incision in the 8th intercostal space at the subscapularis angle and the scapular line, respectively, for placement of a endoscopic grasper and scalpel for dividing the esophagus, and the last 5-mm incision in the 3rd intercostal space at the anterior axillary line as the third working port for pulling of the lung and esophageal exposure. The mediastinal pleuron was cut along the esophagus longitudinally to separate the esophagus. The arch of the azygos vein was divided and the vein was cut with an endoscopic vein vascular stapler or tissue clamp. The esophagus was then pulled and separated along the surgical plane, and the surrounding lymph nodes, adipose tissue around the esophagus, subcarinal lymph nodes and lymph nodes next to the recurrent laryngeal nerve on both sides were also resected completely. The esophagus was exposed down to the diaphragmatic hiatus and up to the neck.
For the abdominal operation, laparotomy or laparoscopic incision was made to both groups to separate the stomach, which was pulled via the transesophageal bed to the neck position for resection of the affected esophagus and connection of the esophagus-stomach anastomosis. The patients were admitted to ICU after surgery, and received anti-inflammatory, hemostatic, phlegm-resolving treatment and nutritional support, as well as continuous infusion of morphine to relieve pain.
Statistical analysis
The statistical indicators included operative time, intraoperative blood loss, number of dissected chest lymph nodes, postoperative complication rate and so on. Long-term follow-up was carried out after surgery to compare the overall survival and survival time. The statistical analysis was performed in GraphPad Prism 5.0. Data between the two groups were compared using Mann Whitney test, and survival analysis was conducted using Log-rank test. P<0.05 was considered significantly different.
Results
All operations were successful in both groups. An incidence of intraoperative bleeding was observed in the VAMS group, with which the surgery was completed following an additional right chest incision for bleeding control. The average time was 43.91 minutes for thoracic surgery, with a median of 50 minutes. The average blood loss was 115.16 mL, with a median of 100 mL. The number of dissected chest lymph nodes was 511, with an average of 4.69/cases. Thoracic lymph node metastasis was observed in 2 cases, with a positive rate of 1.8%. There were 12 cases of anastomotic leakage, one case of mediastinal chyle, seven cases of arrhythmia, and nine cases of hoarseness after surgery, and all were cured and discharged following symptomatic treatment.
The thoracic surgery lasted an average of 76.15 minutes for the VATS group, with a median of 80 minutes; blood loss was 144.5 mL, with a median of 150 mL. The number of dissected chest lymph nodes was 506, with an average of 8.72/cases. Thoracic lymph node metastasis was observed in 2 cases, with a positive rate of 3.4%. There were seven cases of anastomotic leakage, two cases of chylothorax, two cases of arrhythmia, one case of pulmonary embolism, and one case of postoperative thoracic bleeding which required a second surgery to stop. All the subjects with complications were cured and discharged following symptomatic treatment. There was one case of postoperative stump fistula leading to perioperative death due to septic shock.
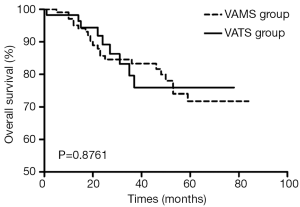
Comparing the two groups, there was no significant difference in postoperative complications (P=0.7284) or the incidence of anastomotic fistula (P=0.8373). The VAMS group was favorable in terms of operative time (P<0.001) and blood loss (P<0.001), and a significantly larger number of chest lymph nodes were dissected in the VATS group compared with the VAMS group (P<0.001). Long-term follow-up in both groups revealed no significant difference in the overall survival (P=0.876; Figure 1).
Discussion
Minimally invasive esophageal surgery has been developing rapidly in just over a decade (6,7,11). As the continuous improvement of the technology and application techniques, the potential of becoming a preferred option to conventional surgery has gradually appeared (12). Since thoracoscopic esophagectomy still takes the traditional approach, despite reduced surgical injury, it still undermines the integrity of the pleural cavity, which is intolerable for certain patients. In recent years, we have adopted the video-assisted mediastinoscopy for the treatment of esophageal cancer, and achieved good outcomes (13). Similar reports have preliminarily demonstrated the feasibility, safety and long-term efficacy of mediastinoscopic resection of esophageal cancer (11-13). Due to limited space and vision under mediastinoscopy, it has remained controversial as to whether lymph node dissection can be accomplished with microscope. Therefore, there are still no unified, definite standards for the indication for mediastinoscopic esophageal resection (12,13). This study compared the efficacy of video-assisted mediastinoscopic and thoracoscopic esophageal surgery with an attempt to summarize the indications for the mediastinoscopic esophageal resection.
Due to limited vision and space, mediastinal endoscopic resection demands for more stringent indications, and therefore it was not suitable for cases with significant tumor invasion or evident mediastinal lymph node involvement. Previous studies have demonstrated that for patients with early esophageal cancer (T2 and before), video-assisted mediastinoscopy can achieve similar therapeutic effect as thoracotomy (11,14). There was no difference in the long-term efficacy for patients with stage T1 after surgery in the both groups. This indicates that esophageal cancer at the T1 stage as confirmed by preoperative endoscopic ultrasound and chest enhanced CT scan, without significantly enlarged mediastinal lymph nodes, can be treated with VAMS esophageal resection, with which a similar therapeutic effect can be achieved as thoracoscopic surgery.
Some investigators reported that in patients with non-small cell lung cancer, cervical mediastinoscopy can be used to dissect all mediastinal lymph nodes except groups 9 and 4L, including the lymph nodes near bilateral recurrent laryngeal nerve (8,15-17). In our experience, the amplification effect of video-assisted mediastinoscopy was conducive to fine operation and thus the detection rate of enlarged mediastinal lymph nodes for complete resection, helping to achieve the purpose of cure. Our results also demonstrated that thoracoscopic surgery has an advantage in the number of dissected mediastinal lymph nodes. Hence, there needs to be a larger-scale multi-center, prospective study to draw more scientific conclusions for mediastinal lymph node dissection under mediastinoscopy.
During neck operation, injury to the recurrent laryngeal nerve is likely to occur. In the VAMS group, there were nine cases of hoarseness, though all of them were healed spontaneously afterwards. In our experience, the use of suction cautery is associated with a high likelihood of recurrent laryngeal nerve injury. Therefore, when separating the upper esophagus during the later part of the surgery, the instrument should be avoided and changed to ultrasonic electrotome for the operation.
In conclusion, T1N0M0 esophageal cancer is the surgical indication for mediastinoscopic resection. Mediastinoscopic esophageal resection is done through the mediastinal pathway, which does no injury to the pleural cavity. Therefore, despite the controversy over mediastinal lymph node dissection, this technique can be considered as the preferred option for patients with poor pulmonary and cardiac function or a history of pleural disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chen W, Zheng R, Zhang S, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res 2013;25:10-21. [PubMed]
- Chen W, He Y, Zheng R, et al. Esophageal cancer incidence and mortality in China, 2009. J Thorac Dis 2013;5:19-26. [PubMed]
- Saglam S, Arifoglu A, Saglam EK, et al. Neoadjuvant hyperfractionated-accelerated radiotherapy with concomitant chemotherapy in esophageal cancer: phase II study. J Gastrointest Oncol 2013;4:380-7. [PubMed]
- Lu J, Tao H, Song D, et al. Recurrence risk model for esophageal cancer after radical surgery. Chin J Cancer Res 2013;25:549-55. [PubMed]
- Ling TC, Kang JI, Slater JD, et al. Proton therapy for gastrointestinal cancers. Transl Cancer Res 2012;1:150-8.
- Tapias LF, Morse CR. Minimally invasive ivor lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014;218:1130-40. [PubMed]
- Navarro-Ripoll R, Córdova H, Rodríguez-D’Jesús A, et al. Cardiorespiratory Impact of Transesophageal Endoscopic Mediastinoscopy Compared With Cervical Mediastinoscopy: A Randomized Experimental Study. Surg Innov 2014. [Epub ahead of print]. [PubMed]
- Binţinţan V, Gutt CN, Mehrabi A, et al. Gas-chamber mediastinoscopy for dissection of the upper esophagus. Chirurgia (Bucur) 2009;104:67-72. [PubMed]
- Mimatsu K, Oida T, Kawasaki A, et al. A novel technique of mediastinoscopy-assisted esophagectomy with a flexible laparoscope and endoscopic overtube. Surg Laparosc Endosc Percutan Tech 2010;20:e44-6. [PubMed]
- Wu B, Xue L, Qiu M, et al. Video-assisted mediastinoscopic transhiatal esophagectomy combined with laparoscopy for esophageal cancer. J Cardiothorac Surg 2010;5:132. [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Venissac N, Pop D, Mouroux J. Video-assisted mediastinoscopy as a therapeutic tool. Surg Endosc 2009;23:2466-72. [PubMed]
- Bonavina L, Incarbone R, Bona D, et al. Esophagectomy via laparoscopy and transmediastinal endodissection. J Laparoendosc Adv Surg Tech A 2004;14:13-6. [PubMed]
- Pop D, Venissac N, Mouroux J. Video-assisted mediastinoscopy improved radical resection for cancer in transhiatal esophagectomy. J Thorac Cardiovasc Surg 2007;133:267-8. [PubMed]
- Zieliński M. Transcervical extended mediastinal lymphadenectomy: results of staging in two hundred fifty-six patients with non-small cell lung cancer. J Thorac Oncol 2007;2:370-2. [PubMed]
- Binţinţan VV, Mehrabi A, Fonouni H, et al. Evaluation of the combined laparoscopic and mediastinoscopic esophagectomy technique. Chirurgia (Bucur) 2009;104:187-94. [PubMed]
- Ikeda Y, Niimi M, Kan S, et al. Mediastinoscopic esophagectomy using carbon dioxide insufflation via the neck approach. Surgery 2001;129:504-6. [PubMed]


