Nocturnal pulse rate and symptomatic response in patients with obstructive sleep apnoea treated with continuous positive airway pressure for one year
Background
Obstructive sleep apnoea (OSA) is the most common form of sleep-disordered breathing (1), it contributes to increased mortality rates secondary to associated cardiovascular (2) and metabolic risks (3), with cognitive deficits impairing work efficiency (4). It is characterised by repetitive episodes of nocturnal apnoeas or hypopnoeas with arousal from sleep leading to an increased sympathetic tone (5).
Continuous positive airway pressure (CPAP) is the most effective available treatment for moderate to severe OSA (6), reducing apnoeas, hypopnoeas and, in symptomatic responders, daytime symptoms like sleepiness (7). However, not every patients benefits symptomatically (8) and the long-term impact of CPAP on arterial blood pressure and cardiovascular risks is not entirely understood: while the acute effects of CPAP on blood pressure have been clearly demonstrated, long term outcomes are still equivocal (9). Key mechanisms linking sleep-disordered breathing and cardiovascular problems are likely to be multi-factorial and potentially involve sympathetic activity (10), intermittent hypoxia (11), inflammation (12), hyper-coagulation (13) and endothelial dysfunction (14).
Heart rate is a predictor of hypertension and a major risk factor for cardiovascular mortality in non-OSA populations, despite considerable differences between the genders (heart rate-to-blood pressure correlation) (15). Increased heart rate in OSA may derive from a high sympathetic tone and previous studies have shown that an increased sympathetic activation is associated with OSA in men but the evidence is lacking in women. Sympathetic activation leads to dysregulation of the circadian heart rate rhythm, potentially affecting systemic arterial blood pressure, heart rate and cardiac arrhythmias (16). Non-invasive monitoring and recording of nocturnal pulse rate (PR), a surrogate of heart rate (17), is safe, widely available and could be used to better understand the sympathetic activity in OSA (18,19).
It is important to predict long-term symptomatic outcomes and associated cardiovascular risks in patients with OSA when commencing them on CPAP therapy. We hypothesised that nocturnal PR in patients with OSA, measured during the diagnostic night as a marker for sympathetic tone, can help to predict long term changes in daytime sleepiness and blood pressure that are associated with CPAP therapy.
Patients and methods
The study was approved by the local institution’s review board (registration number: 2012-2773). Patients referred to the Sleep Disorders Centre at Guy’s & St. Thomas’ NHS Foundation Trust, London, UK for suspected OSA were screened using nocturnal pulse oximetry (Pulsox 300i, Konica Minolta sensing Inc, Hachioji, Tokyo, Japan) for two consecutive nights at home. We analysed the pulse and oxygen trace with PULSOX DS-5 software (Anandic Medical Systems, Feuerthalen, Zurich/Switzerland). The device gives the moving average of the last eight PRs at one-second intervals. We selected the night with the better quality and longer recording time for further analysis.
Inclusion criteria for the OSA group were clinical presentation suspicious of OSA plus a 4% oxygen desaturation index (4% ODI) ≥5/h. We also included a matched control group of patients without OSA. The inclusion criteria were an ODI <5/h and a pulse rise index (PRI) lower than 20/h. The PRI is defined as an increase in the PR by more than six beats per minute and these events are counted over a one hour period. Patients without significant OSA can exhibit an increased PRI caused by periodic limb movements or other underlying conditions leading to autonomic arousals and an increase sympathetic tone. We therefore decided to exclude subjects from the control group if they had an abnormal high PRI. On the other hand, patients with OSA have commonly an elevated PRI and, therefore, we did not use this criterion for the OSA group. We selected the threshold of five events/hour for the 4% ODI in line with recommendations for the apnoea-hypopnoea-index and the National Institute of Clinical Excellence (NICE) guidance (7).
Exclusion criteria for both groups were ongoing use of heart rate-affecting medications (digoxin, anti-arrhythmic agents, beta-blockers or calcium antagonists, including verapamil and diltiazem), chronic atrial fibrillation and other paroxysmal or permanent arrhythmias, second or third degree atrio-ventricular block, permanent pacemaker, diagnosis of periodic limb movement disease (PLMD), age under 18 and over 70 years old, pregnancy, acute/critical illness (decompensated heart failure or end stage COPD and renal failure), patients admitted to a hospital ward.
Patients who discontinued CPAP over the study period were excluded, as were those who were lost to follow up and those with corrupted pulse oximetry data. The pulse oximetry data of the patients with a clear history of snoring, witnessed apnoeas and excessive daytime sleepiness [EDS, as assessed by the Epworth sleepiness scale (ESS) >10 points] (20), were subsequently reviewed by a physician experienced in sleep medicine and the diagnosis of OSA was confirmed (4% ODI of ≥15/h, or ≥5/h plus elevated ESS) and CPAP titration was initiated. CPAP treatment was started after 2-weeks of pressure titration using an AutoSet device (APAP, S8/S9, ResMed Ltd, Sydney, Australia). Optimal CPAP pressure was identified as the 95th percentile of APAP pressure. Upon initiation of CPAP therapy, patients attended an induction by specialised sleep technicians, blood pressure and weight were recorded. Patients with treated OSA were followed to assess weight, blood pressure, ESS and adherence to CPAP therapy at one year. To study the PR trend throughout the night, we calculated the difference between mean PR of the last and the first hour of the nocturnal recording. In order to be consistent throughout the study we decided that the first and last hour of pulse oximetry was likely not to represent sleep. The patients were further analysed in a subgroup according to ascending (positive ΔPR) or descending (negative ΔPR) PR.
Blood pressure was measured with an automated device (Mindray VS-800, Medical International Limited, Shenzhen, China) according to the American Heart Association reccomendations (21). Subjective daytime sleepiness was assessed using the ESS (20). The primary end point was to compare mean PR and ΔPR of the first screening night of pulse oximetry with the change of blood pressure at one year in patients with OSA treated with CPAP. Our secondary end point was to determine whether changes in daytime sleepiness (ΔESS) differed between patients with different PR responses (ΔPR) over one year of CPAP treatment. Adherence to treatment and compliance were measured at follow up as the number of hours per night and the overall percentage of CPAP usage.
Statistical analysis
Statistical analysis of the data was performed using GraphPad Prism (Version 5.02, GraphPad Software Inc, San Diego, California/USA). Data are reported as mean (standard deviation, SD), if not otherwise indicated, correlations were stated including the 95% confidence interval (CI). Following testing for normality, similarity of two means was compared using student’s t-test and Chi-square or Fisher’s exact tests statistics in case of normal distribution; otherwise Wilcoxon signed-rank test was used. Within group variables were compared using student t-test for paired data. Spearman’s rank correlation coefficient was calculated for non-normally distributed variables. For all tests, a value of P<0.05 was considered significant (22).
Results
In the final analysis, we included 58 patients with OSA [36 males, age 49.4 (1.2) years, weight 109.7 (3.6) kg] and 57 without OSA [34 males, age 46.4 (1.7) years, weight 95.0 (7.0) kg].
The two groups were matched regarding gender (P=0.850), age (P=0.161) and weight (P=0.105). Patients with OSA were more sleepy (P<0.0001). The ODI, PRI, mean PR, mean saturation and ΔPR were significantly different between the groups (P<0.0001, respectively; Table 1).
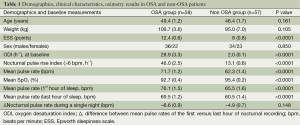
Full table
Mean PR was higher in male subjects with OSA compared to men without OSA [71.3 (1.6) vs. 58.5 (1.7) min–1, P<0.0001]; in the control group female subjects had a higher mean PR than men [68.1 (2.1) vs. 58.4 (1.7) min–1, P<0.001].
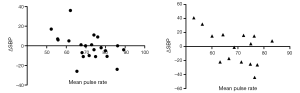
In the OSA group, ΔPR correlated negatively with mean oxygen saturation (r=–0.39, 95% CI, –0.59 to –0.13, P<0.01), as did the mean PR (r=–0.4, 95% CI, –0.6 to –0.14, P<0.01). The mean PR and the change in systolic blood pressure (ΔSBP) at one year follow up correlated negatively (r=–0.42, 95% CI, –0.66 to –0.1, P<0.05; Figure 1). There was no correlation between ΔPR and systolic (r=–0.049, P=0.77) or diastolic blood pressure (DBP) (r=–0.146, P=0.38).
Following diagnosis of OSA and commencing on CPAP treatment, patients in the OSA group at one year follow up had used the device for 5.0 (1.9) hours per night with an average total percentage of nights used of 63.0% (36.7%).
In a subgroup analysis of patients with OSA who were grouped depending on the change in nocturnal PR (positive vs. negative ΔPR) we found that, at baseline, the weight of the patients with a positive ΔPR (n=11) was higher [124.4 (26.6) vs. 104.8 (23.1) kg, P<0.05] and the patients were more sleepy compared to the patients with a negative ΔPR (n=47) [10.5 (5.8) vs. 6.9 (4.9) points in the ESS, P<0.05]. At one year follow up, there was no significant difference between the groups with respect to SBP or DBP), but the level of daytime sleepiness had significantly reduced in those with a negative ΔPR compared to those with a nocturnal rise in the PR [ΔESS –5.8 (5.1) vs. –0.8 (7.2) points, P<0.05, Tables 2 and 3].
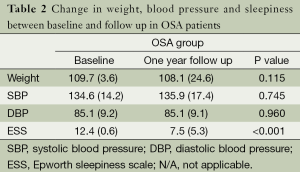
Full table
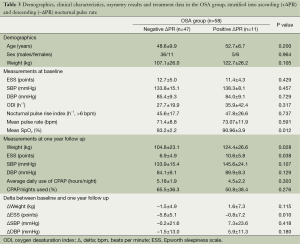
Full table
Discussion
The mean nocturnal PR of patients with OSA is elevated when compared to those who do not experience nocturnal sleep disruption and the nocturnal change in PR (ΔPR) is associated with a better symptomatic response to CPAP treatment at one year. In OSA patients, mean nocturnal PR correlated inversely with ΔSBP in the one year follow up. While most of the OSA patients demonstrated a reduction in nocturnal PR (negative ΔPR), those with a rise in nocturnal PR (positive ΔPR) revealed lower mean oxygen saturations at baseline and had symptomatically responded less to CPAP treatment at one year.
Clinical significance
Various factors may influence nocturnal PR. Non-rapid eye movement (REM) sleep is associated with reduced sympathetic nerve activity, reduction in blood pressure and lower heart rate, whilst REM sleep is associated with more irregular ventilation and increases in blood pressure and heart rate (5). Our data support the hypothesis that an increased PR in patients with OSA reflects an enhanced sympathetic activation (23) and therefore contributes to the development of hypertension in OSA patients, representing a major risk factor for cardiovascular and non-cardiovascular morbidity and death (15).
Understanding nocturnal PR and its trend during the night appears to be important for patients with sleep apnoea, but has not been considered yet as a diagnostic or prognostic tool. Nocturnal pulse oximetry is frequently used for the diagnosis of sleep apnoea in European-based sleep centres (24), but more recently its use has also been assessed elsewhere (25). Complementing the analysis of the recorded oxygen trace by considering the trend of the nocturnal PR might help to early stratify sleep apnoea patients who are likely to symptomatically respond to treatment.
Kawano et al. (26) have recently shown that mean heart rate over 24 hrs correlates well with the apnoea-hypopnea-index (AHI), nocturnal SpO2 and arousal index in patients with OSA. In our study, the change in nocturnal PR (ΔPR) and mean PR correlated inversely with mean oxygen saturation. This observation links the tonic chemoreflex activation to increased sympathetic activity in patients with OSA.
We found a difference between the mean nocturnal PR in male OSA patients and matched male subjects in the control group. However, there was no difference in female subjects in the two groups, presumably because heart rate in the control group was higher in females, as had been described in previous studies (27). Moreover, the association between SBP and PR in male and female subjects suggests a possible gender-specific difference in the OSA population (28) (Figure 1). A significant association between sleep-related breathing disorders and hypertension had previously been reported only in males (29), while a questionnaire-based, case-controlled study found that the association of sleep-related breathing disorders and hypertension was independent of BMI in women, but not in men (30). Nevertheless, gender-specific differences have been poorly evaluated and reliable data in large cohorts of women are needed.
Our findings are consistent with results by Sanner and colleagues who showed that elevated heart rate predicts a better CPAP effect on blood pressure (31) and may indicate the higher sympathetic impact on heart rate and blood pressure (32). However, in their study no consideration was given to exclude patients treated with heart rate affecting drugs.
In our study, patients with a fall in nocturnal PR (negative ΔPR) demonstrated a significant decrease in weight at follow up. This finding might help to identify patients that benefit more from CPAP in terms of reducing their overall cardiovascular risk. In order to confirm this hypothesis the change in nocturnal PR needs to be validated prospectively in a larger cohort of patients with OSA.
The benefit of CPAP on blood pressure and body weight is still under debate: in unselected OSA patients, CPAP has modest effects on blood pressure. In contrast, patients with more severe OSA and difficult-to-control hypertension seem to benefit significantly (33,34). Similarly, an impact of CPAP therapy on body weight has not been clearly demonstrated: a recent randomised sham-controlled study concluded that reducing visceral obesity in men with OSA cannot be achieved by CPAP alone (35).
Limitations
Our study was an observational cohort in which we were limited to pre-recorded data; we cannot rule out that our final analysed sample of patients was not representative for OSA patients. Both groups of patients included in the analysis were obese and this could have affected heart rate because of an increased sympathetic and reduced vagal activity (36). In this study we investigated for possible correlations between nocturnal PR in patients with OSA and weight change, following initiation of CPAP. We acknowledge that in our study we only measured weight and not BMI. However, it has to be considered that height in the adult population does not regularly change over time. The measurement of weight at baseline and at the follow up appointments will therefore indicate the same amount of change in the parameter that would have been found if BMI had been measured. A prospective study with height and BMI measurements could help to better match groups. Moreover, ΔPR may not accurately reflect PR when asleep. However, the availability of nocturnal pulse oximetry enhances the clinical usefulness of this method. Blood pressure was only recorded during the initial CPAP titration and at one year follow up; it could therefore be argued that the CPAP titration period may have affected the baseline blood pressure. Compliance to CPAP treatment in the OSA patients was limited and this could have further affected the outcome parameters. However, our study highlights the fact that there are sparse data on the change in blood pressure of OSA patients after CPAP treatment has continued for more than six months (9).
Conclusions
This study reveals that nocturnal PR is increased in OSA patients reflecting an elevated sympathetic tone. A nocturnal reduction in PR during the diagnostic night is associated with an improved symptomatic response to CPAP therapy at one year.
Further prospective studies are needed to better assess the long term effect of CPAP on blood pressure and body weight and to confirm the possible predictive role of mean PR and ΔPR in OSA patients. However, recording nocturnal PR and ΔPR is a widely available method and may help to determine OSA patients with a symptomatic response to CPAP and those with an increased cardiovascular risk.
Acknowledgements
We are grateful for the statistical support of Professor Janet Peacock (King’s College London, UK).
Sponsors: Guy’s & St. Thomas’ NHS Foundation Trust.
Disclosure: The authors declare no conflict of interest.
References
- Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. [PubMed]
- He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 1988;94:9-14. [PubMed]
- Martinez D, Klein C, Rahmeier L, et al. Sleep apnea is a stronger predictor for coronary heart disease than traditional risk factors. Sleep Breath 2012;16:695-701. [PubMed]
- Philip P, Sagaspe P, Lagarde E, et al. Sleep disorders and accidental risk in a large group of regular registered highway drivers. Sleep Med 2010;11:973-9. [PubMed]
- Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303-7. [PubMed]
- Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [PubMed]
- National Institute for Health and Clinical Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. No. of guidance 139, 2008; London: National Institute for Health and Clinical Excellence.
- Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 2007;30:711-9. [PubMed]
- Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens 2012;30:633-46. [PubMed]
- Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol 2000;119:189-97. [PubMed]
- Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 2010;174:156-61. [PubMed]
- Del Rio R, Moya EA, Parga MJ, et al. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur Respir J 2012;39:1492-500. [PubMed]
- Terada S, Koyama T, Watanabe H, et al. Abnormal coagulation and platelet profile in patients with obstructive sleep apnea syndrome. Int J Cardiol 2011;146:423-5. [PubMed]
- Bruno RM, Rossi L, Fabbrini M, et al. Renal vasodilating capacity and endothelial function are impaired in patients with obstructive sleep apnea syndrome and no traditional cardiovascular risk factors. J Hypertens 2013;31:1456-64. [PubMed]
- Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens 1997;15:3-17. [PubMed]
- Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897-904. [PubMed]
- Khandoker AH, Karmakar CK, Palaniswami M. Comparison of pulse rate variability with heart rate variability during obstructive sleep apnea. Med Eng Phys 2011;33:204-9. [PubMed]
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet 2002;359:204-10. [PubMed]
- Gil E, Orini M, Bailón R, et al. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol Meas 2010;31:1271-90. [PubMed]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. [PubMed]
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697-716. [PubMed]
- Fisher RA. eds. Statistical Methods and Scientific Inference. New York: Hafner, 1956.
- Narkiewicz K, Somers VK. Interactive effect of heart rate and muscle sympathetic nerve activity on blood pressure. Circulation 1999;100:2514-8. [PubMed]
- Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep 2012;35:757-67. [PubMed]
- Vázquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax 2000;55:302-7. [PubMed]
- Kawano Y, Tamura A, Watanabe T, et al. Influence of the severity of obstructive sleep apnea on heart rate. J Cardiol 2010;56:27-34. [PubMed]
- Lavie-Nevo K, Pillar G. Evening-morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens 2006;19:1064-9. [PubMed]
- Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung 2007;185:67-72. [PubMed]
- Hedner J, Bengtsson-Boström K, Peker Y, et al. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J 2006;27:564-70. [PubMed]
- Marrone O, Bonsignore MR, Fricano L, et al. Gender and the systemic hypertension-snoring association: a questionnaire-based case-control study. Blood Press 1998;7:11-7. [PubMed]
- Sanner BM, Tepel M, Markmann A, et al. Effect of continuous positive airway pressure therapy on 24-hour blood pressure in patients with obstructive sleep apnea syndrome. Am J Hypertens 2002;15:251-7. [PubMed]
- Kufoy E, Palma JA, Lopez J, et al. Changes in the heart rate variability in patients with obstructive sleep apnea and its response to acute CPAP treatment. PLoS One 2012;7:e33769. [PubMed]
- Obstructive Sleep Apnoea Syndrome. Report of a joint Nordic project. Finnish Office for Health Care Technology Assessment. Obstructive Sleep Apnoea Syndrome. Report of a joint Nordic project. Helsinki: Finnish Office for Health Care Technology Assessment (FinOHTA) 2007.
- Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;CD001106. [PubMed]
- Hoyos CM, Killick R, Yee BJ, et al. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax 2012;67:1081-9. [PubMed]
- Grassi G, Vailati S, Bertinieri G, et al. Heart rate as marker of sympathetic activity. J Hypertens 1998;16:1635-9. [PubMed]


