Pulmonary adenocarcinoma: implications of the recent advances in molecular biology, treatment and the IASLC/ATS/ERS classification
Introduction
The recent years have witnessed many a breakthrough in the management of various malignancies. These breakthroughs have been possible not only due to innovations in treatment methodologies, but also due to enhanced understanding of pathological, molecular and genetic basis of cancers. In this day and age, almost every malignancy is amenable for sub-classification, which has been possible due to an enhanced understanding of the heterogeneous nature of malignancies. These classifications help not only in prognostic stratification, but also in guiding specific treatments.
Across the years, multiple new therapeutic agents have been made available for the management of lung cancer. However, this comes with a caveat—all agents cannot be applied in all patients of lung cancer. The histology dependence within non-small cell lung cancer (NSCLC) was demonstrated by the fact that the use of pemetrexed was associated with no benefits among patients with squamous cell histology (1). Additionally, it was understood that the use of bevacizumab was associated with risks of life-threatening hemorrhage when used in patients with squamous cell histology (2). Thus, the importance of sub-classifying ‘NSCLC’ into more specific histological subtypes is now very justifiable.
More than half of all NSCLC diagnosed worldwide happen to be adenocarcinomas (3). However, adenocarcinomas in themselves are not all the same, since a lot of heterogeneity exists in terms of pathological and molecular features. In the recent years, the runaway success had with the use of imatinib for molecular targeting of the mutated BCR-ABL gene led to the initiation of efforts by various research groups to develop targeted therapies for many malignancies, including lung cancer (4). While the pre-clinical studies with the use of tyrosine kinase inhibitors against mutated epidermal growth factor receptors (EGFR)-TKIs appeared to offer good prospects for the treatment of lung cancer, it was later understood via clinical trials that the benefit with EGFR-TKIs was not applicable to all patients of NSCLC. Sub-group analyses of initial phase-III trials implied better benefits among specific populations, such as those patients with adenocarcinoma histology, patients of East Asian origin, never-smokers, and women (5). This selective specificity of benefit with EGFR-TKIs fueled further efforts into molecular profiling, which revealed that the benefit with EGFR-TKIs was specific to patients with EGFR gene mutations, which in turn were more likely to be found in tumors with adenocarinoma histology. In addition to EGFR-targeting, recent understanding regarding various other driver mutations in lung adenocarcinomas has led to intense research efforts towards development of novel agents towards other mutations. Recent success has also been observed with the use of crizotinib, an agent which targets mutations involving ALK-gene mutations (6). However, beyond EGFR and ALK targeted approaches, the progress in targeting other known mutations has rather been minimal, and remains to be a focus of ongoing research.
The advances in management of lung adenocarcinoma have not necessarily been restricted to molecular breakthroughs, but are also attributable to advances in multiple other disciplines including pathology, radiology, oncology and surgery. The prior classification of lung cancer, which was developed by the World Health Organization (WHO) was mainly a pathological classification, developed by panels mainly consisting of pathologists (7). Thus, there was a need for a modern classification which would encompass multidisciplinary perspectives in the classification of lung adenocarcinomas. Working in this direction, there was a combined effort by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS) and the European Respiratory Society (ERS). The joint effort by the IASLC, ATS and the ERS culminated in the development of comprehensive guidelines (published in the year 2011) for the classification of lung adenocarcinomas, which not only provided classification guidelines, but also provided guidance towards good practice and recommendations for further research (8).
In this review, the recent understandings with respect to the molecular biology and molecular targeted therapy in lung adenocarcinomas are discussed. Also, a concise summary of the important recommendations in the recent IASLC/ATS/ERS classification of lung adenocarcinoma is also provided. Lastly, potentially emerging issues which could affect the management protocols of lung adenocarcinomas in the coming years are also discussed.
Molecular biology of lung adenocarcinoma
The ‘hallmarks of cancer’ imply abilities of limitless replicative potential, self sufficiency at growth, anti-apoptotic potential, sustained angiogenesis and the potential for invasion and metastasis. These mentioned ‘abilities’ are acquired due to dysregulation of signaling pathways. For a malignancy to occur, the underlying genetic mechanisms could include oncogene activation (via gene amplification, rearrangements, and point mutations), or via loss of tumor suppressor gene function (by loss of heterozygosity, or by epigenetic transcriptional silencing). Both these mechanisms have been understood to be involved in the etio-pathogenesis of lung cancer (9,10).
This section describes the recent advances in relation to the advances in molecular biology of oncogene activation leading to lung cancer. Though loss of tumor suppressor gene function as an etiology of lung cancer was understood much before the concept of oncogene activation was even postulated, there have been greater efforts towards understanding the molecular biology behind oncogene activation, mainly owing to the feasibility of molecular targeted therapy.
Research into the mechanisms involved in oncogene activation led to the discovery of the phenomenon of ‘oncogene addiction’. This recently described phenomenon states that certain tumors rely upon one single dominant oncogene for the purpose of initiation, growth and survival, and that the inhibition of this specific oncogene leads to the regression of the particular tumor. Proof for the concept can be had from the myriad examples of success had from molecular targeting, such as with the use of imatinib for the inhibition of the BCR-ABL fusion gene in chronic myeloid leukemia, or the use of traztuzumab for the treatment of human epidermal receptor 2 (HER2) over-expressing breast cancer (6,11,12).
The oncogenes involved in ‘oncogene addiction’ are also termed as ‘driver oncogenes’. These driver oncogenes represent conditional vulnerability in lung cancer—given that the tumor cells are dependent on the aberrant gene function for survival and proliferation. These in a way represent the ‘achilles heel’ of the tumor, given that the phenomenon allows an unique therapeutic opportunity since the targeting of the driver oncogene would lead to specific killing of the ‘oncogene addicted’ tumor cells alone (13,14).
In lung cancer, the commonly activated driver oncogenes include EGFR, kirsten rat sarcoma oncogene (KRAS), HER2, MYC, mesenchymal epidermal transition (MET), EML4-ALK and BCL2. Clinically, EGFR mutations are the most important not only because of them being among the most common mutations in lung adenocarcinoma, but also because of the availability of effective targeted therapies against the same. The translocation mutation EML4-ALK too has garnered enough attention recently because of the availability of crizotinib for targeting. The clinical success in targeted therapies for lung adenocarcinoma has unfortunately for now been restricted to EGFR and ALK mutations, and intense research is however underway to develop novel agents to target other known driver mutations (15-18).
EGFR mutations in lung adenocarcinoma
EGFR belongs to a family of trans-membrane receptor tyrosine kinases, consisting of three other closely related receptors—the HER2, HER3, and the HER4. The EGFR and other members of the family are expressed in various normal tissues of epithelial, mesenchymal and neural origin. Knockout studies in mice have demonstrated that EGFR receptor family is necessary for survival, and is involved in development and maintenance of important organs such as the skin, mucosa, heart, lungs and the central nervous system (19,20).
Given the pivotal role of EGFR in regulating growth, it is not surprising that mutations involving the EGFR are oncogenic. Small molecule EFGR inhibitors are inhibitors of tyrosine kinases, which block the binding of ATP to the tyrosine kinase catalytic domain. The success had with oral EGFR inhibitors has been remarkable enough for them to be approved for first line use in locally advanced NSCLC with EGFR mutated status.
The exact worldwide prevalence of EGFR mutations among adenocarcinoma patients is difficult to estimate. The main reason being that EGFR mutations’ occurrence seems to vary with ethnicity- being very uncommon among blacks, with an incidence of <20% of all NSCLC among whites, and with an incidence of 20-40% of all NSCLC among patients of East Asian origin. It must however be noted that the available statistics mostly estimate the incidence among NSCLC as a whole, and it is only recently since the publication of the IASLC/ATS/ERS classification guidelines that the importance of sub-classifying NSCLC into more specific subtypes has been realized. Nevertheless, if a population of non-smokers, with adenocarcinoma histology, and Asian origin were to be considered, then the likelihood of the tumor harboring EGFR mutations would be well over 60% (5,21-23).
The mutations affecting the kinase domain region (located from exon 18 to 21) of the EGFR gene are regarded as ‘activating mutations’ since these mutations result in constitutive kinase activity of the receptor kinase (24). The most frequent of these activating mutations involve various mutations at exon 19 amounting for 45% of the cases, while point mutations (mostly the L858R mutation) account for 40% of the cases. The remaining 15% is comprised of various point or insertion mutations in exons 18 to 21 (25,26). There is evidence to state that differential sensitivity to TKIs among patients with regards to the type of EGFR mutation (27,28).
Targeting the mutant EGFR
Prior to realization of the potential with EGFR inhibition, NSCLC was a ‘clinical entity’ amenable to treatment with cytotoxic chemotherapy, and this offered modest response rates of 25-30% and a median survival (MS) of about a year (29). In 2004, three independent research groups reported that EGFR mutations in lung cancer were amenable for targeting by TKIs (30-32).
Initial studies testing the efficacy of EGFR-TKIs had involved NSCLC patients after prior treatment with chemotherapy. These early studies such as the BR.21 and the INTEREST trials were conducted in unselected patients of NSCLC without regards to their EGFR mutation status. Despite the fact that these trials involved both mutated and non-mutated patients, there was evidence of benefit with the use of gefitinib/erlotinib in comparison to placebo or chemotherapy. The BR.21 trial involved 731 previously treated NSCLC (unselected mutation status) allotted to treatment with erlotinib versus placebo. Erlotinib was better in terms of both progression free survival (PFS) (2.2 vs. 1.8 months, P<0.001) and MS (6.7 vs. 4.7 months, P<0.001) (33). The phase III trial included 1,433 previously treated NSCLC patients (not selected as per EGFR INTEREST status). The study compared second line treatment with gefitinib versus docetaxel. The study indicated non-inferiority of gefitinib to docetaxel (34).
Though the BR.21 and the INTEREST trials used second line treatment with EGFR-TKIs in NSCLC patients untested for EGFR mutations, there were many studies which did not support this approach in comparison to the use of second line chemotherapy. The phase-III trial V-15-32 was conducted in Japan to assess the efficacy of second line treatment with gefitinib in comparison to second line chemotherapy with docetaxel. This study failed to show non-inferiority of gefitinib to docetaxel among NSCLC patients unselected as per histology or EGFR status (35).
From sub-group analysis of previous trials, reports were made that EGFR-TKIs were of greater effect in certain populations—such as in patients of Asian origin, non-smokers and those with adenocarcinoma histology. Thus, trials were then on designed based on patients selected by background characteristics. The IPASS trial was conducted with an intention to assess the efficacy of first-line treatment with gefitinib in previously untreated Asian non-smokers with advanced adenocarcinoma. The study involving 1,217 patients compared gefitinib versus chemotherapy with carboplatin-paclitaxel. Though the overall PFS was better with gefitinib, there was controversy in interpretation since the survival curves of the two groups crossed each other. Thus, it was concluded that for the entire study population, the PFS with gefitinib was better than chemotherapy at 12 months, while it was worse than chemotherapy at 3 months. However, when sub-group analysis was performed with regards to EGFR mutation status, it was clearly delineated that the use of gefitinib offered better PFS among patients with mutated EGFR (HR =0.48, P<0.001) and that the use of chemotherapy offered better PFS for patients with non-mutated EGFR (HR =2.85, P<0.001). Thus the IPASS study was revolutionary in that it demonstrated the benefits of gefitinib among EGFR mutated patients, while also demonstrating the non-effectiveness of gefitinib among those without EGFR mutations (5,36).
The phase-III OPTIMAL trial was initiated with the intention to compare the PFS benefit with erlotinib versus gemcitabine-carboplatin, when used as first line treatment in Chinese patients with EGFR mutated advanced NSCLC. The median PFS was better with erlotinib in comparison to chemotherapy (13.1 vs. 4.6 months, P<0.0001). Comparable results were also obtained from the European trial EURTAC which compared erlotinib versus chemotherapy for first line treatment of EGFR mutated European patients (37,38). The American Cancer Society has released a provisional clinical opinion that patients being considered for first-line therapy with an EGFR-TKI should be tested for EGFR mutations (39).
In addition to the fact the EGFR-TKIs offer better PFS in comparison to chemotherapy in EGFR mutated patients, the quality of life outcomes also were much better for patients treated with EFGR-TKI in comparison to chemotherapy. Obvious reasons include the ease of oral administration of TKIs, and the relative rarity of severe systemic side-effects with TKIs. The tolerability of EGFR-TKI have made it an agent feasible for addition to best supportive care among EGFR mutated adenocarcinoma patients with very poor performance status, who may otherwise be unfit for any other form of treatments. Occasionally dramatic regressions have been noticed, leading to a consequential improvement in performance status, offering a new lease of survival for a sub-population otherwise endowed with no hope (40).
Resistance to EGFR-TKI agents
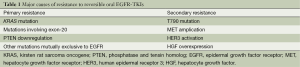
Resistance to TKI, can be of two sorts—intrinsic or acquired. Intrinsic resistance (also known as primary resistance) occurs among tumors which are inherently resistant to EGFR-TKIs. On the other hand, acquired resistance, also known as secondary resistance implies the development of non-response to EGFR-TKIs among patients who had initially responded to treatment (41). The causes of intrinsic and acquired resistance have been enlisted in Table 1.
Full table
Molecular causes for primary resistance include the presence of KRAS mutation, phosphatase and tensin homolog (PTEN) down-regulation, or the presence of exon-20 mutation. KRAS encodes a GTPase downstream of EGFR, and thus the activation of KRAS activates downstream signaling pathways independent of EGFR (42,43). Another cause for intrinsic resistance to EGFR-TKIs could be the down-regulation of PTEN expression. PTEN is an important negative regulator of the PI3K pathway which promotes proliferation and is anti-apoptotic. Thus, down-regulation of PTEN causes unhindered growth and survival (44). Patients with exon-20 mutations are known to show a lower response rate (of about 25%) to EGFR-TKIs in comparison to those patients with mutations affecting exons 19 or 21 (45,46). Additionally, a deletion polymorphism in the gene encoding the pro-apototic protein BIM (BCL2-like 11) is also likely to confer primary TKI resistance (47).
The issue of acquired (secondary) resistance is not new. The earliest identified phenomenon was the development of BCR-ABL T315I mutation which conferred resistance to imatinib (48). With regards to EGFR inhibitors, the most common cause (accounting for more half of cases) is the development of the T790M mutation, which occurs due to substitution of the amino-acid threonine at position 790 with methionine in exon-20. This leads to a lack of inhibitor specificity at the ATP binding site, leading to increased ATP affinity, the cause behind drug resistance (49-51). It is to be noted that the T790M mutation can occasionally be the cause of primary resistance too. Hepatocyte growth factor (HGF) receptor (MET) amplification reportedly accounts for about 20% of cases with secondary resistance (52). Other documented causes for secondary resistance include HGF over-expression and HER3 activation (53,54).
The understanding regarding the possible development of secondary resistance among patients being treated with EGFR-TKI warrants thoughtful consideration towards the use of re-biopsy for secondary molecular assessments (46).
Management of resistance
Gefinitib and erlotinib, both are reversible EGFR inhibitors. The use of irreversible dual EGFR-HER2 inhibitors has been investigated for patients who develop secondary resistance to EGFR-TKIs. The LUX-1 study compared afatinib versus best supportive care in 585 patients whose disease progressed after one or two lines of chemotherapy and at least 12 weeks of erlotinib/gefitinib treatment. Though a PFS difference favored treatment with afatinib, there was no overall survival benefit. The overall response rate for afatinib was 11% (55). A phase-III trial (LUX-lung 5) has been initiated to assess the role of afatinib with weekly paclitaxel versus investigator’s choice of single agent chemotherapy following afatinib monotherapy in patients failing treatment with gefitinib/erlotinib (56).
Dacomitinib is another irreversible pan-HER inhibitor which too has been tried among patients who have developed resistance to erlotinib/gefitinib. In a study involving 188 unselected patients of NSCLC who had failed two lines of prior chemotherapy, the use of dacomitinib was compared with erlotinib. Median PFS and objective response rates tended to be better with dacomitinib. Importantly, among KRAS mutated patients (primary resistance to gefitinib and erlotinib) the benefit with dacomitinib seemed to be much better (57,58).
EGFR testing: newer methods
While direct sequencing DNA testing for mutations requires biopsy material, newer sequencing techniques have arrived, which offer ability to make cytology-based testing. Many of these PCR techniques have been already tested in clinical trials, such as in the Japanese trials NEJ 001 to 003 (59-61).
A recent study described the sensitivity and comparability among five commercially available next generation sequencing (NGS) techniques. All techniques were able to perform better than the older direct sequencing technique, while being able to detect mutation types at >1% mutant deoxyribonucleic acid (DNA), with successful analysis rates of 91.4-100%. Thus given the remarkable sensitivity of NGS techniques, it can be stated that cytology derived DNA is a viable alternative to formalin fixed paraffin embedded tissues samples for analysis of mutations (29,62-64).
Significant of EGFR copy number
In addition to testing the significance of EGFR mutation, the IPASS study also tested the significance of EGFR gene copy number. This sub-group analysis tended to imply that EGFR mutated patients with increased copy number tended to have better PFS increments with gefitinib in comparison to those without increased EGFR copy number (6,65).
KRAS mutations in lung adenocarcinoma
KRAS mutations have been detected in about 30% of NSCLC, with the majority occurring in codons 12 and 13. The mutations result in constitutive activation of RAS signaling, which is present downstream of EGFR pathway. The presence of KRAS mutations have been the cause for a significant proportion of cases with primary resistance to EGFR-targeted therapy not only in lung cancer, but also in other malignancies such as colo-rectal and pancreatic cancers (41,66-70).
While EGFR mutations are more common in the young and in non-smokers, KRAS mutations tend to be noticed among the elderly and in heavy smokers (71). Though the quest to develop clinically effective KRAS targeted therapy has been mostly futile until this date, the importance of KRAS testing in lung adenocarcinomas lies in the fact that KRAS mutation positivity is an indicator of primary resistance to EGFR-TKIs.
KRAS mutation testing can be used as an alternative to EGFR testing, since KRAS and EGFR mutations are mostly mutually exclusive. The KRAS mutations are confined to only three codons, thus lowering the costs of mutation sequencing. Further, patients negative for both EGFR and KRAS mutations may harbor the chromosomal translocation EML4-ALK, which may be present in about 3-6% of patients (23,72,73).
ALK rearrangements in lung adenocarcinoma
Many anaplastic lymphoma kinase (ALK) fusion oncogenes are known to occur as driver mutations. The most common among ALK fusion genes in lung adenocarcinoma is the echinoderm microtubule associated protein like 4 (EML4)-anaplastic lymphoma kinase (ALK) translocation fusion gene, which was first described as recently as in 2007 (74-76). The relative prevalence of ALK translocation mutations among lung adenocarcinoma patients is rather low (3-6%) in comparison to EGFR and KRAS mutations. However, if a population of adenocarcinoma patients were to be chosen as per a negative smoking history, and negative EGFR/KRAS mutation status, then the prevalence of ALK rearrangement mutations is significant (77).
Crizotinib is a TKI which is ATP-competitive for the receptor tyrosine kinases ALK and MET (78). Initial studies demonstrated an objective response rate of 56% and a median PFS of 10 months, which was impressive given the use in a population of patients pre-treated with prior chemotherapy regimens. This led to an accelerated approval for advanced ALK positive adenocarcinoma (79,80).
Although most patients with ALK-positive lung adenocarcinoma derive initial clinical benefit from crizotinib, the benefit is relatively short-lived because of the development of acquired resistance. The causes behind secondary resistance to crizotinib are a matter of ongoing research. As of now, only isolated cases have been studied with regards to the molecular basis behind secondary resistance. The currently reported mechanisms include L1152R and L1196M mutations. In vitro experiments have indicated that EGFR activation could be another mechanism behind crizotinib resistance (81,82).
Katayama et al. evaluated 18 patients with acquired resistance to crizotinib. A diverse variety of secondary mutations distributed throughtout the ALK tyrosine kinase domain region were noted in about 25% of the patients. Also, ALK fusion gene amplification could be behind secondary resistance (76).
MET amplication in lung adenocarcinoma
MET receptor is a tyrosine kinase which can be activated by its ligand, HGF. Abnormal MET activity can be a result of various mechanisms such as MET gene amplification, HGF over-expression or MET gene mutation. While evidence implicating the effects of MET gene mutation in carcinogenesis is rather sparse, there is ample evidence that over-expression of HGF, or that MET gene amplification is associated with poor prognosis in lung adenocarcinoma (83-85).
Further, MET amplification is purported to be the cause of secondary resistance to EGFR-TKIs in up to 20% of patients with EGFR mutations. Thus, MET inhibition has emerged as an important target since its inhibition can potentially restore sensitivity to EGFR-TKIs (86-88).
Various methods of targeting the MET pathway include the use of small molecule inhibitors of the MET receptor, or by the use of monoclonal antibodies against HGF. Small molecule inhibitors of MET are of two main categories—selective inhibitors such as tivantinib, and non-selective inhibitors such as crizotinib, foretinib, and cabozantinib. Various ongoing trials have been focusing not only upon MET targeting, but also upon dual-MET-EGFR blockade (89-91).
VEGF targeting in lung adenocarcinoma
VEGF is an endothelial cell specific ligand which in important in regulating angiogenesis in normal and tumor tissues. VEGF-pathway is amenable to be targeted by three broad approaches: (I) via the use of monoclonal antibodies to target VEGF; (II) via the use of VEGF-Receptor inhibitors such as aflibercept; and (III) by the use of small molecular TKIs such as sunitinib and sorafenib to target the tyrosine kinase domain of VEGF-receptor (92-94).
The Eastern Cooperative Oncology Group (ECOG) protocol 4,599 and the European AVAIL were two large phase-III trials which were pivotal in the gaining of approval for bevacizumab in lung cancer (33,95,96). Histology dependence for the use of bevacizumab has been identified, with current indication for bevacizumab in lung cancer limited to ‘non-squamous’ histologies.
Though effective in certain populations, the use of bevacizumab is undeniably associated with significant treatment related morbidities and mortalities. Moreover, there have been no trials which have compared EGFR-TKIs versus bevacizumab, given that both agents seem to be used in a patient population with overlapping characteristics.
Aflibercept is a recombinant fusion protein used to target the VEGF, which has been investigated for use in platinum- and erlotinib- resistant pulmonary adenocarcinoma. Despite promise in early trials, the results in phase-III trials has been rather discouraging (97,98). Pazopanib, sunitinib, mosatenib and sorafenib are TKIs which act by inhibiting the VEGFR, PDGFR and the RAS/RAF pathways. These agents are yet to be proven for efficacy and safety in phase-III trials (99-102).
Other targets
Unfortunately, the progress in respect of targeting the various other known oncogenic mutations in lung adenocarcinoma has rather been sparse. The development of targeted therapies against several other oncogenic mutations in pulmonary adenocarcinoma is an intense field of ongoing research with multiple preclinical & early trials attempting to develop agents to target mutations involving BRAF, ROS1, RET, HER2, DDR2, FGFR1, ILGF-1 and various others (103-110).
THE IASLC/ATS/ERS classification of lung adenocarcinoma
The past decade has witnessed the emergence of important understandings with regards to the management of lung cancer. The most important being the demonstration of ‘histology dependence’ for therapy with pemetrexed and bevacizumab, that these two agents are to be avoided in squamous cell carcinoma. The next most important finding was the discovery that activating mutations, such as EGFR and ALK are markers for response to specific TKIs. Further, many advances have occurred in the management of lung cancer, in terms of pathology, pulmonology, oncology and surgery. While the 2004 edition of the WHO classification of lung cancer was primarily a ‘pathological classification’, the need for a multidisciplinary classification was felt. The IALSC, the ATS and the ERS joined forces to set-up an international multidisciplinary panel consisting of pathologists, clinicians, surgeons, molecular biologists and radiologists. A systematic review of 11,368 citations, of which 312 articles were retrieved for full text review, was performed, and meetings were held to discuss the development of recommendations along with the new classification. All the recommendations were graded for their quality and strength by means of the grades of recommendation, assessment, development & evaluation (GRADE) approach. The new classification and the recommendations were published in the Journal of Thoracic Oncology, which is the official journal of the IASLC. An accompanying editorial was also published in the European Respiratory Journal, which is the official journal of the ERS (8,111).
This section of the review intends to provide a summary of the salient new recommendations in the 2011 IASLC/ATS/ERS lung adenocarcinoma classification. Readers are strongly recommended that they refer to the official publication of the classification published in the Journal of Thoracic Oncology for full details of the classification, the accompanying recommendations and the discussions supporting the recommendations (8).
Classification
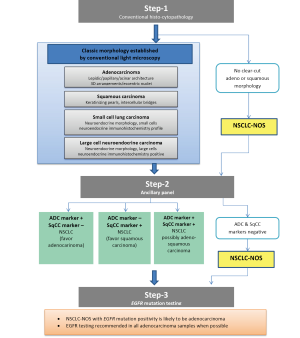
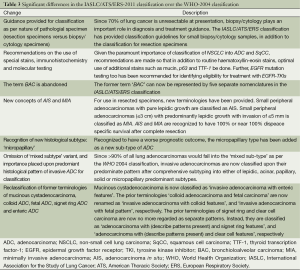
The conspicuous difference between the WHO-2004 and the IASLC/ATS/ERS-2011 classification is that the IASLC/ATS/ERS classification provides guidelines for classification based upon the nature of specimen. Up to about 70% of lung cancer patients present in advanced stages such that they are inoperable at diagnosis, these patients are generally treated with a non-surgical approach (chemotherapy, radiotherapy and molecular therapy) and resection specimens are hence unavailable. Underscoring this reality, the IASLC/ATS/ERS classification has provided standardized guidelines for pathological classification based on cytology or small biopsy samples (Figure 1). In addition, there are comprehensive guidelines for pathological classification of resection specimens too (Table 2). The other main difference from the WHO-2004 classification is that the IASLC/ATS/ERS classification has discarded the usage of the term ‘bronchioloalveolar carcinoma’ (BAC) since the tumors formerly classified as BAC can now fall under five distinct diagnoses (Tables 3,4). In addition to acinar, papillary and solid patterns, two new patterns, namely the ‘micropapillary’ and the ‘lepidic’ patterns have been added. The term ‘mixed subtype adenocarcinoma’ is not used in the IASLC/ATS/ERS classification.

Full table
Full table

Full table
While the WHO-2004 classification primarily was based upon the use of haematoxylin-eosin stains for pathological classification, the new IASLC/ATS/ERS classification attempts to integrate additional histopathological techniques such as mucin, thyroid transcription factor-1, and p-63 staining- all with an intention to be more capable of classifying NSCLC into adenocarcinoma and squamous cell carcinoma, given the implications upon treatment planning. Further, the IASLC/ATS/ERS classification recommends the use of EGFR mutation testing to further sub-classify adenocarcinoma patients so as to assess feasibility of treatment with EGFR-TKIs. A summary of new features in the IASLC/ATS/ERS classification over the WHO-2004 classifications are presented in the Tables 3,4.
Recommendations
The use of histopathology forms the backbone of the new classification, even though efforts have been made in to integrate perspectives from clinical, radiological, molecular and surgical disciplines. In addition to providing classification guidelines, the new classification also provides a number of important evidence based recommendations.
One of the most important recommendations is the abandoning of the term ‘BAC’. After publication of the 2004 edition of the WHO classification of lung cancer, the term BAC used to cause major confusions in interpretations, since it could be used for a rather wide range of entities- ranging from small solitary noninvasive peripheral lung tumors [which could have 100% 5-year disease specific survival (DSS)], to advanced invasive lesions with lepidic patterns (which could have low survival prospects) (112,113).Thus, the old term BAC has now been abandoned, and the spectrum of new nomenclatures which could fall into the old ‘BAC’ terminology is enlisted in Table 4.
Multiple studies had shown 100% DSS for patients with small solitary peripheral adenocarcinomas with pure lepidic growth (114-118). Similarly, 100% or near 100% 5-year DSS was observed for patients with minimally invasive tumors, if a cut off of 5 mm depth of invasion was used (119-123). Keeping these findings into consideration, the new classification has introduced two new concepts, namely ‘adenocarcinoma in situ’ (AIS) and ‘minimally invasive adenocarcinoma’ (MIA).
AIS refers to small (≤3 cm) localized adenocarcinoma with pure lepidic growth (growth restricted to neoplastic cells along preexisting alveolar structures), while lacking other patterns, and also marked by the absence of intraalveolar tumor cells, stromal, vascular, or pleural invasion. MIA refers to small (≤3 cm) solitary adenocarcinoma with a predominantly lepidic pattern and ≤5 mm maximum size of invasion. In case of multiple microinvasive areas in one tumor, the size of the largest invasive area, in the largest dimension should be measured. It must be noted that the MIA concept cannot be applied if the tumor contains foci of necrosis, or if there is any invasion of lymphatics, pleura or blood vessels.
Since it was noted that more than 90% of lung adenocarcinomas would fall into the WHO-2004 category of ‘mixed subtype’ adenocarcinoma, many independent groups assessed the feasibility of classifying lung adenocarcinomas as per the predominant histologic subtype. This methodology offers prospects of correlations between various histologic patterns with molecular features and clinical behavior (124-129). Thus, the term ‘mixed-subtype adenocarcinoma’ is not used in the IASLC/ATS/ERS-2011 classification. Semi-quantitative recording of patterns in 5% increments is adopted, and while the single most predominant pattern defines the diagnosis, it must be kept in mind that the percentages of the non-predominant patterns should also be recorded.
Though the WHO-2004 classification had mentioned the ‘micropapillary pattern’ of lung adenocarcinoma in the discussion section, there was no inclusion of this pattern as a formal subtype. Since tumors with a predominant micropapillary patterns are noted to be associated with poor prognosis, akin to adenocarcinomas with predominant solid pattern, the IASLC/ATS/ERS-2011 classification includes the ‘micropapillary pattern’ as a new major histological subtype (130-133). It is possible that even the presence of small amounts of micropapillary patterns may confer poor prognosis, and this is likely to be a focus of future research (134,135). It is noteworthy at this stage that a recent study described two types of micropapillary patterns that can occur in lung adenocarcinoma- namely the more common ‘aerogenous micropapillary component’ (AMPC) (with tumor cells floating in the alveolar spaces) and the ‘stromal invasive micropapillary component’ (SMPC) (with tumor cells found invading the stroma). Though the relative prevalence of SMPC (3.4%) was much smaller than the prevalance of AMPC (17.7%), it was observed on multivariate analysis that poorer DFS for stage I adenocarcinoma patients was associated with SMPC and not AMPC (136).
Rationale is also provided for the classification of invasive adenocarcinomas into mucinous and nonmucinous types. This is because that mucinous and nonmucinous adenocarcinomas seem to have major differences in terms of clinical behavior, pathology, and mutational status. While mucinous tumors are likely to show a strong correlation with KRAS mutations, non-mucinous tumors are more likely to display EGFR mutations (137-141).
While a summary of the recommendations is provided in Table 5, the authors again remind the readers to refer to the original manuscript published by Travis et al. in the Journal of Thoracic Oncology for full details and discussion (8).

Full table
Research recommendations enlisted in the IASLC/ATS/ERS-2011 classification
Members involved with the IASLC/ATS/ERS classification project had initiated research protocols in an attempt to develop data to test the IASLC/ATS/ERS guidelines. Studies conducted to validate the guidelines included projects on small biopsies, grading, molecular-histological correlations, AIS, MIA and major histological patterns (142-145). Though the classification guidelines were written backed by the best evidence available at the time of drafting, there is a recognized need for further validation of the guidelines with more data. Indeed, the IASLC/ATS/ERS classification has also provided guidance on further research with regards to the classification and correlation with radiology, molecular biology and treatment of lung adenocarcinomas.
Pathology research prospects
- Minimally invasive adenocarcinoma criteria—Since the MIA definition utilizes 5 mm as size cut-off for invasion size, the optimal method of measuring the size of the invasion component has to be established. Clarity also needs to be established as to the procedure with a tumor is associated with multiple foci of invasion.
- Impact of the nature of invasive component in MIA upon survival—It is to be established if the nature of the invasive component affects the disease free survival, since it is possible that micropapillary and solid patterns could possibly adversely affect survival.
- Impact of secondary patterns in invasive carcinoma—Since the guidelines use the most predominant pattern for sake of classification, it is to be investigated as to how the secondary patterns may affect prognosis. Given that micropapillary pattern is likely to adversely affect prognosis even when present in very small proportions of 1-5%, there is a possibility that more aggressive patterns may have a significant effect upon prognosis even if they are not the predominant pattern (146,147).
- Ideal grading system—Since the current guidelines do not recommend any specific grading system, it may be necessary to investigate the utility of grading done by architectural grading versus nuclear grading.
- Patterns in metastatic sites—The prognostic implications of histological patterns in metastatic sites when compared to primary site is to be investigated.
- Utility of frozen sections—The ability of pathologists to be able to distinguish in situ disease versus invasive disease upon examinations on frozen section is to be investigated.
- Impact of ancillary diagnostic methods—The use of special stains with mucin, and with immunohistochemistry with TTF-1, p63 etc. have been suggested so as to reduce the frequency of NSCLC-NOS diagnoses. It is to be investigated as to how the classification by the use of special stains may possibly affect clinical outcomes, since existing data is based upon conventional light microscopy alone. Also, the utility of additional markers such as napsin-A is to be investigated.
Clinical and surgical research prospects
- Adenocarcinoma in non-smokers—The clinical, epidemiological, molecular and histological characteristics of adenocarcinoma occurring in never-smokers is to be investigated.
- Impact of histological pattern in early stage lung cancer upon adjuvant therapy—It is to be investigated as to whether the histological patterns present affect the need for adjuvant chemotherapy in early stage lung cancer after resection. Given that the micropapillary pattern is predictive of a higher potential for metastases, patients of the same stage with aggressive histological features may require more intense treatment.
- Sub-lobar resections for some early adenocarcinomas—Though lobectomy is the standard surgical procedure for tumors less than 2 cm in size, it is to be investigated as to whether sub-lobar resections can offer equivalent survival for selected patients with radiologically detected ground-glass opacities which could be predictive of AIS and MIA.
- Less morbid approaches for early stage lung adenocarcinoma—It is to be investigated to assess the results with thoractotomy versus video-assisted thoracic surgery (VATS) with regards to morbidity and survival outcomes.
- Can lymph-nodal dissection be omitted in some early stage adenocarinomas? Recent data suggests that some very early stage adenocarcinomas (especially the ground glass opacity lesions diagnosed radiologically) may not require lymph-nodal dissection (148).
- Protocol for multi-focal adenocarcinomas—There is need to evolve a treatment algorithm for management of multiple lesions with regards to nature, number, size, locations, synchronous versus metachronous lesions, and primary versus metastatic lesions.
Molecular research prospects
- Histology-molecular correlations to be assessed- While certain histologies are likely to be associated with certain mutations (e.g., Signet ring cell histology with EML4-ALK mutations, mucinous adenocarcinoma with KRAS, non-mucinous adenocarinoma with EGFR), further research is required to assess the frequency and strength of these associations (137-141).
- Micro-RNA, genomics, and proteomics—evaluation is required to assess if micro-RNA, genomics and proteomics testing can provide information regarding risk stratification and outcome prediction in lung adenocarinomas.
Radiology research prospects
- Radiological measurement of tumor—The ideal method of measurement is to be established. With regards to part-solid and part-ground glass lesions, the importance of the relative proportions of the solid and ‘ground glass components’ is to be established.
- Possibility of use of computed tomography (CT) attenuation data analysis to differentiate between multiple-primaries versus metastases to be investigated
- Attempts to establish molecular correlations with radiological findings such as ground glass opacities and standardized uptake value (SUV) on positron emission tomography to be embarked upon.
- Impact of the new classification upon CT-based screening for lung cancer to be evaluated.
Potential implications of the IASLC/ATS/ERS classification upon staging
The recommendations within the IASLC/ATS/ERS classification has potential implications upon future editions of the tumor-nodes-metastases (TNM) staging for lung cancer. First of all, it may be feasible to provide separate staging systems for lung cancer as per histology, similar to the current version of staging for esophageal carcinoma which has separate staging systems for squamous and adenocarcinomas. Secondly, it may be possible to integrate the new concepts of AIS and MIA into TNM staging systems by away similar to the current staging system for breast cancer, by incorporation of the terms Tis (for in-situ lesions) and Tmi (for microinvasive lesions). It is also to be seen if radiological assessments of tumor volume with regards to the invasive and the non-invasive components by volumetric measurements of the solid and ground-glass components could be used in staging for patients who may be treated by non-surgical approaches, such as with radical stereotactic ablative radiotherapy (SABR). However, much data needs to be accumulated before the drafting of the next edition of the TNM-AJCC staging for any changes to be incorporated (149-151).
Potential controversial issues with the IASLC/ATS/ERS classification
It is to be noted that the IASLC/ATS/ERS classification in itself acknowledges weaknesses and also has provided guidelines for further research to strengthen or refute the recommendations provided within the classification system. However, there are some issues which confer a degree of ambiguity on all possible interpretations on classification made using the IASLC/ATS/ERS guidelines.
Given the complexity introduced into the histological sub-typing, it is likely that reproducibility with regards to inter-observer variations among pathologists will be a rather serious issue. The most likely areas of confusion will be with regards to assigning a diagnosis of lepidic versus acinar pattern, and in differentiating the micropapillary and the papillary patterns (152,153).
Given that the IASLC/ATS/ERS classification has deleted the ‘mixed-subtype’ category in favor of typing by the most-predominant histological pattern, important questions remain to be answered. Given that solid and micropapillary subtypes are considered to be of poor prognostic significance, it is likely that the presence of these patterns even in small proportions could adversely affect prognosis. As in the case with the use of the Gleason score in prostate cancer, it is to be investigated if there is a rationale in providing weightage to non-predominant patterns. However, the IASLC/ATS/ERS does encourage research on the issue of non-predominant patterns as part of its pathology research recommendations.
Other questions to be answered include the relative importance of architectural grading in comparison to nuclear grading, the impact of inflammation or stromal desmoplasia upon determination of invasion size, and the best possible methodology for quantifying depth/size of invasion.
Future prospects in lung adenocarcinoma
MicroRNAs (miRNA)—potential in lung cancer diagnosis, prognostification and treatment
miRNA are non-coding RNA which act as post-transciptional regulators of gene expression (154). Importance in research on miRNAs is justified since miRNAs are known to be involved in the ability to regulate multiple genetic pathways, having both pro- and anti-oncogenic abilities (155). miRNAs are thought to have great potential in terms of diagnosis, prognostification and treatment of lung cancers.
The use of miRNA detection algorithms for lung cancer diagnosis is an attractive concept since it allows methods of non-invasive lung cancer diagnosis using blood miRNA expression. miRNA-25 and 223 were identified as biomarkers of NSCLC, and then the detection of a pattern characterized by overexpression of miRNAs-155, 182 and 197 could be used to separate patients with lung cancer from cancer free subjects. miRNA based diagnosis has also raised possibility of the use of sputum for the purpose of lung cancer diagnosis (156). Various i signatures can also possibly used to differentiate between adenocarcinoma and squamous cell carcinomas. Recent studies have demonstrated the effectiveness of using miRNA-205 as a marker for squamous cell lung cancer. Further, it is likely that miRNA signatures will help in the differentiation of primary lung tumors from lung metastases. Also, various studies have enumerated miRNA signatures which could possibly help in the prognostification of lung cancer patients. Much research however needs to be put in efforts of standardizing the potential protocols for the use of miRNAs in diagnosis and prognostification in routine clinical use. Since miRNAs are known to be involved in pro- and anit-oncogenic effects, efforts are underway to develop clinically feasible techniques to utilize miRNA based treatments for the treatment of lung cancer. Potential approaches include the inhibition of oncogenic miRNAs, as well as in enhancing the functions of tumor suppressive miRNAs (157-160).
Cancer stem cell (CSC) research
Treatment with chemotherapy, radiotherapy or molecular targeted therapy often downsizes the tumor, many times to even cause clinical complete remissions. However, a significant majority of these tumors ultimately recur—either locally or at distant sites. The CSC hypothesis explains this phenomenon. Given that the bulk of the tumor can be eradicated by cytotoxic agents, it is possible that cancer stem cells remain quiescent, hence conferring themselves protection against cytotoxic agents and ionizing radiation- which basically are active against dividing cells. These surviving CSCs reactivate later on, leading to relapse (161).
Regulation of CSCs is likely mediated by the Hedgehog, Wnt and the Notch signaling pathways (162). Eradication of CSCs would lead to prospects of ‘cure’ after complete remissions after standard therapies have been achieved. Efforts are on to develop methods of detecting CSC related biomarkers for clinical use (163-165). With regards to targeting of CSC associated pathways, clinical progress has been steady- with ongoing experiments using cyclopamine to target the hedgehog pathway; and with promising potential with the use of gamma-secretase inhibitors to target the notch pathways (166,167). Also, it is postulated that CSCs can be eradicated by the use of very high doses of radiotherapy, as would occur with the use of SABR in eligible patients (168).
Potential with SABR
Stereotactic-irradiation combines highly conformal delivery of radiation to selected volumes at large doses per fraction, with the treatment completed typically within one to five fractions. The radiobiological equivalence of doses delivered by stereotactic-irradiation (often beyond 80-100 Gy) is much higher in comparison to the doses achievable by conventional fractionation. The recent emergence of various technological innovations with regards to image guidance and precision radiotherapy has allowed the routine use of hypofractionated stereotactic radiotherapy in almost any site in the body. At the high fraction sizes used in stereotactic-irradiation, evidence suggests the role of various radiobiological mechanisms of actions, which are not traditionally associated with conventional radiotherapy. These include induction of endothelial damage, cell membrane damage, organelle damage, ceramide pathway activation and eradication of dormant tumor cells (168-171).
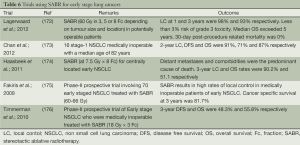
SABR is also referred to by other acronyms such as stereotactic body radiotherapy (SBRT), extracranial sterotactic RT (ESRT) and fractionated sterotactic radiotherapy (FSRT). Initial studies of SABR in lung cancer involved early stage NSCLC among patients who were inoperable due to medical reasons. It was noted that the approach was comparable to surgery in terms of results, while being very tolerable. Further, the use of SABR is not associated with a significant risk of immediate mortality unlike with thoracic surgery. Also, the local control rates are excellent, consistently exceeding 95% at 1-year, and median overall-survival consistently exceeds 5-year. SABR is now being tested by a number of trials for operable lung cancers too. An outline of the recent trials addressing the use of SABR in early stage NSCLC is provided in Table 6 (172-177).
Full table
It must be emphasized here that SABR at present can only be applied to small target volumes as seen in stage-I and II tumours, for patients who are inoperable for any reasons. Also, given that the use of SABR will cause destruction of the tumor, all required pathological testing should be established by means of small biopsy/cytology testing prior to the initiation of SABR.
Emerging prognostic and predictive indicators
Various histopathological prognostic factors for lung adenocarcinoma have already been well described in existing literature. Factors such as intratumoral vessel invasion, visceral pleural invasion, perineural invasion, mitotic index, Ki-67 labelling index, histological grade, presence of tumor necrosis etc. have been established as being prognostic for tumor recurrence after complete resection (178-183).
Advances in nuclear medicine have also led to the emergence of new prognostic indicators such as with a high SUV in a FDG-PET scan being indicative of poor differentiation and increased propensity for lymph-nodal involvement.
The advances in genetic profiling has led to the emergence of prognostic molecular assays for various malignancies- such as with the OncotypeDX (TM) and MammaPrint (TM) for breast cancer—which are designed to be predictive of relapse after surgery for early breast cancer, and to help assist in deciding the necessity for adjuvant chemotherapy. Similar molecular profiling systems are also available for colon cancer. Though prognostic molecular assays for lung adenocarcinoma is not yet commercially available, the feasibility for the same was described recently by Kratz et al., who utilized a 14-gene expression assay to predict patient at high risk of mortality after surgical resection (184-187). If validated in large trials, it is likely that personalized decisions regarding the use adjuvant chemotherapy after surgical resection of early stage lung adenocarcinoma can be feasible.
In addition to prognostic factors, recent years have seen the emergence of ‘predictive factors’. Which though not indicative of patient prognosis are useful in being able to ‘predict’ responsiveness to specific agents. The excision repair cross-complementing group 1 (ERCC1) and the ribonucleotide reductase M1 (RRM1) genes are naturally involved in repair of DNA, and overexpression of these genes are thought to reduce efficacy of platinum compounds and gemcitabine, respectively. Also, advances in molecular profiling have led to the prediction of sensitivity to, or resistance for specific molecular targeting agents (188-190).
Conclusions
There has been a deluge of new discoveries in relation to the management of lung adenocarcinomas. The realization of the importance of sub-classifying NSCLC into more specific histologies was first felt due to the availability of histology suited therapies such as pemetrexed and bevacizumab. Then, the availability of effective molecular targeted therapies for specific mutations has now made it important for inculcating molecular testing as part of investigation protocols for lung adenocarcinomas. The recent IASLC/ATS/ERS classification of lung adenocarcinoma is a step forward, in that it has made efforts towards inclusion of perspectives not only from the advances in pathology, but also from those in molecular biology, radiology, oncology and surgery. The IASLC/ATS/ERS classification comes with major procedural amendments over the WHO-2004 for the classification of lung adenocarcinomas. The IASLC/ATS/ERS has also provided important guidelines for research, so as to help gain evidence towards strengthening the recommendations put forth in the classification. In the coming years, it is possible that accelerated developments in the field of molecular biology, genetic profiling, radiology, nuclear medicine, surgery, radiotherapy and chemotherapy will bring forth major changes in the way lung adenocarcinoma is diagnosed and treated.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [PubMed]
- Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184-91. [PubMed]
- Kim HSIH, Choi YS, Kim K, et al. Surgical resection of recurrent lung cancer in patients following curative resection. J Korean Med Sci 2006;21:224-8. [PubMed]
- Stegmeier F, Warmuth M, Sellers WR, et al. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Ther 2010;87:543-52. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Travis WD, Brambilla E, Muller-Hermelink HK, et al. eds. Pathology and Genetics. Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Lorusso G, Rüegg C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochem Cell Biol 2008;130:1091-103. [PubMed]
- Goldman JM, Melo JV. Chronic myeloid leukemia--advances in biology and new approaches to treatment. N Engl J Med 2003;349:1451-64. [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [PubMed]
- Weinstein IB. Cancer. Addiction to oncogenes-the Achilles heal of cancer. Science 2002;297:63-4. [PubMed]
- Perez R, Crombet T, de Leon J, et al. A view on EGFR-targeted therapies from the oncogene-addiction perspective. Front Pharmacol 2013;4:53. [PubMed]
- Bronte G, Rizzo S, La Paglia L, et al. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev 2010;36 Suppl 3:S21-9. [PubMed]
- Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 2013;31:1097-104. [PubMed]
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol 2011;12:175-80. [PubMed]
- Rossi A, Maione P, Colantuoni G, et al. Recent developments of targeted therapies in the treatment of non-small cell lung cancer. Curr Drug Discov Technol 2009;6:91-102. [PubMed]
- Yano S, Kondo K, Yamaguchi M, et al. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res 2003;23:3639-50. [PubMed]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol 2007;190:1-65. [PubMed]
- Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res 2005;11:1167-73. [PubMed]
- John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene 2009;28:S14-23. [PubMed]
- Bulman W, Saqi A, Powell CA. Acquisition and processing of endobronchial ultrasound-guided transbronchial needle aspiration specimens in the era of targeted lung cancer chemotherapy. Am J Respir Crit Care Med 2012;185:606-11. [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor in lung cancer. Nat Rev Cancer 2007;7:169-81. [PubMed]
- Johnson BE, Janne PA. Epidermal growth factor receptor mutations in patients with non–small cell lung cancer. Cancer Res 2005;65:7525-9. [PubMed]
- Nurwidya F, Murakami A, Takahashi F, et al. Molecular mechanisms contributing to resistance to tyrosine kinase-targeted therapy for non-small cell lung cancer. Cancer Biol Med 2012;9:18-22. [PubMed]
- Zhu JQ, Zhong WZ, Zhang GC, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 2008;265:307-17. [PubMed]
- Antonicelli A, Cafarotti S, Indini A, et al. EGFR-targeted therapy for non-small cell lung cancer: focus on EGFR oncogenic mutation. Int J Med Sci 2013;10:320-30. [PubMed]
- Kobayashi K, Hagiwara K. Epidermal growth factor receptor (EGFR) mutation and personalized therapy in advanced nonsmall cell lung cancer (NSCLC). Target Oncol 2013;8:27-33. [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci 2004;101:13306-11. [PubMed]
- Shepherd FA, Rodrigues Pereira J, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [PubMed]
- Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52. [PubMed]
- Nguyen KS, Neal JW. First-line treatment of EGFR-mutant non-small-cell lung cancer: the role of erlotinib and other tyrosine kinase inhibitors. Biologics 2012;6:337-45. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Keedy VL, Temin S, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 2011;29:2121-7. [PubMed]
- Langer CJ. The “Lazarus Response” in Treatment-Naïve, Poor Performance Status Patients With Non–Small-Cell Lung Cancer and Epidermal Growth Factor Receptor Mutation. J Clin Oncol 2009;27:1350-4. [PubMed]
- Zhang Z, Stiegler AL, Boggon TJ, et al. EGFR-mutated lung cancer: a paradigm of molecular oncology. Oncotarget 2010;1:497-514. [PubMed]
- Hammerman PS, Janne PA, Johnson BE. Resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small cell lung cancer. Clin Cancer Res 2009;15:7502-9. [PubMed]
- Bonanno L, Schiavon M, Nardo G, et al. Prognostic and predictive implications of EGFR mutations, EGFR copy number and KRAS mutations in advanced stage lung adenocarcinoma. Anticancer Res 2010;30:5121-8. [PubMed]
- Chow LML, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett 2006;241:184-96. [PubMed]
- Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res 2008;14:4877-82. [PubMed]
- Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 2011;17:1169-80. [PubMed]
- Ng KP, Hillmer AM, Chuah CT, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 2012;18:521-8. [PubMed]
- An X, Tiwari AK, Sun Y, et al. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res 2010;34:1255-68. [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [PubMed]
- Nakamura T, Sueoka-Aragane N, Iwanaga K, et al. A noninvasive system for monitoring resistance to epidermal growth factor receptor tyrosine kinase inhibitors with plasma DNA. J Thorac Oncol 2011;6:1639-48. [PubMed]
- Fujita Y, Suda K, Kimura H, et al. Highly sensitive detection of EGFR T790M mutation using colony hybridization predicts favorable prognosis of patients with lung cancer harboring activating EGFR mutation. J Thorac Oncol 2012;7:1640-4. [PubMed]
- Cappuzzo F, Jänne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009;20:298-304. [PubMed]
- Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor–activating mutations. Cancer Res 2008;68:9479-87. [PubMed]
- Shi F, Telesco SE, Liu Y, et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A 2010;107:7692-97. [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [PubMed]
- http://clinicaltrials.gov/show/NCT01085136
- Ramalingam SS, Boyer M, Park K, et al. Randomized phase 2 study of PF299804, an irreversible human epidermal growth factor receptor (egfr) inhibitor, versus (v) erlotinib (e) in patients (pts) with advanced non-small cell lung cancer (nsclc) after chemotherapy (ct) failure: quantitative and qualitative benefits [abstract 365]. Ann Oncol 2010;21:viii122.
- Halmos B, Powell CA. Update in lung cancer and oncological disorders 2010. Am J Respir Crit Care Med 2011;184:297-302. [PubMed]
- Inoue A, Kobayashi K, Usui K, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 2009;27:1394-400. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 Study. J Thorac Oncol 2012;7:1417-22. [PubMed]
- Goto K, Satouchi M, Ishii G, et al. An evaluation study of EGFR mutation tests utilized for non-small-cell lung cancer in the diagnostic setting. Ann Oncol 2012;23:2914-9. [PubMed]
- Vadakara J, Borghaei H. Personalized medicine and treatment approaches in non-small-cell lung carcinoma. Pharmgenomics Pers Med 2012;5:113-23. [PubMed]
- Collins FS, Hamburg MA. First FDA authorization for next-generation sequencer. N Engl J Med 2013;369:2369-71. [PubMed]
- Chang JW, Liu HP, Hsieh MH, et al. Increased epidermal growth factor receptor (EGFR) gene copy number is strongly associated with EGFR mutations and adenocarcinoma in non-small cell lung cancers: a chromogenic in situ hybridization study of 182 patients. Lung Cancer 2008;61:328-39. [PubMed]
- Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in kras are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol 2005;23:5900-9. [PubMed]
- Pao W, Wang TY, Riely GJ, et al. Kras mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2005;2:e17. [PubMed]
- Jackman DM, Yeap BY, Lindeman NI, et al. Phase ii clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol 2007;25:760-6. [PubMed]
- van Zandwijk N, Mathy A, Boerrigter L, et al. Egfr and kras mutations as criteria for treatment with tyrosine kinase inhibitors: Retro- and prospective observations in non-small-cell lung cancer. Ann Oncol 2007;18:99-103. [PubMed]
- Toyooka S, Mitsudomi T, Soh J, et al. Molecular oncology of lung cancer. Gen Thorac Cardiovasc Surg 2011;59:527-37. [PubMed]
- Subramanian J, Govindan R. Molecular genetics of lung cancer in people who have never smoked. Lancet Oncol 2008;9:676-82. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming eml4-alk fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Yung RC, Otell S, Illei P, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol 2012;120:185-95. [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13-7. [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17.
- Kim HR, Shim HS, Chung JH, et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer 2012;118:729-39. [PubMed]
- Casaluce F, Sgambato A, Maione P, et al. ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC. Target Oncol 2013;8:55-67. [PubMed]
- O’Bryant CL, Wenger SD, Kim M, et al. Crizotinib: a new treatment option for ALK-positive non-small cell lung cancer. Ann Pharmacother 2013;47:189-97. [PubMed]
- Bang YJ. Treatment of ALK-positive non-small cell lung cancer. Arch Pathol Lab Med 2012;136:1201-4. [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [PubMed]
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011;71:6051-60. [PubMed]
- Beau-Faller M, Ruppert AM, Voegeli AC, et al. MET gene copy number in non-small cell lung cancer: molecular analysis in a targeted tyrosine kinase inhibitor naïve cohort. J Thorac Oncol 2008;3:331-9. [PubMed]
- Cheng TL, Chang MY, Huang SY, et al. Overexpression of circulating c-met messenger RNA is significantly correlated with nodal stage and early recurrence in non-small cell lung cancer. Chest 2005;128:1453-60. [PubMed]
- Go H, Jeon YK, Park HJ, et al. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:305-13. [PubMed]
- Wang W, Li Q, Takeuchi S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res 2012;18:1663-71. [PubMed]
- Nakagawa T, Takeuchi S, Yamada T, et al. Combined therapy with mutant-selective EGFR inhibitor and Met kinase inhibitor for overcoming erlotinib resistance in EGFR mutant lung cancer. Mol Cancer Ther 2012;11:2149-57. [PubMed]
- Takeuchi S, Wang W, Li Q, et al. Dual inhibition of Met kinase and angiogenesis to overcome HGF-induced EGFR-TKI resistance in EGFR mutant lung cancer. Am J Pathol 2012;181:1034-43. [PubMed]
- Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer 2009;10:281-9. [PubMed]
- Yano S, Takeuchi S, Nakagawa T, et al. Ligand-triggered resistance to molecular targeted drugs in lung cancer: roles of hepatocyte growth factor and epidermal growth factor receptor ligands. Cancer Sci 2012;103:1189-94. [PubMed]
- Menis J, Levra MG, Novello S. MET inhibition in lung cancer. Transl Lung Cancer Res 2013;2:23-39.
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4-6. [PubMed]
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat 1995;36:127-37. [PubMed]
- Braghiroli MI, Sabbaga J, Hoff PM. Bevacizumab: overview of the literature. Expert Rev Anticancer Ther 2012;12:567-80. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [PubMed]
- Zhang Y, Sun Y, Wang L, et al. Sequential treatment of tyrosine kinase inhibitors and chemotherapy for EGFR-mutated non-small cell lung cancer: a meta-analysis of Phase III trials. Onco Targets Ther 2013;6:1771-7. [PubMed]
- Leighl NB, Raez LE, Besse B, et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol 2010;5:1054-9. [PubMed]
- Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol 2012;30:3640-7. [PubMed]
- Domvri K, Zarogoulidis P, Darwiche K, et al. Molecular Targeted Drugs and Biomarkers in NSCLC, the Evolving Role of Individualized Therapy. J Cancer 2013;4:736-54. [PubMed]
- Kaneda H, Yoshida T, Okamoto I. Molecularly targeted approaches herald a new era of non-small-cell lung cancer treatment. Cancer Manag Res 2013;5:91-101. [PubMed]
- Dienstmann R, Martinez P, Felip E. Personalizing therapy with targeted agents in non-small cell lung cancer. Oncotarget 2011;2:165-77. [PubMed]
- Planchard D. Identification of driver mutations in lung cancer: first step in personalized cancer. Target Oncol 2013;8:3-14. [PubMed]
- Lee J, Ou SH. Towards the goal of personalized medicine in gastric cancer--time to move beyond HER2 inhibition. Part II: Targeting gene mutations and gene amplifications and the angiogenesis pathway. Discov Med 2013;16:7-14. [PubMed]
- Lee J, Ou SH. Towards the goal of personalized medicine in gastric cancer--time to move beyond HER2 inhibition. Part I: Targeting receptor tyrosine kinase gene amplification. Discov Med 2013;15:333-41. [PubMed]
- Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910-8. [PubMed]
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046-51. [PubMed]
- Takezawa K, Okamoto I, Yonesaka K, et al. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res 2009;69:6515-21. [PubMed]
- Minuti G, D’Incecco A, Cappuzzo F. Targeted therapy for NSCLC with driver mutations. Expert Opin Biol Ther 2013;13:1401-12. [PubMed]
- D’Arcangelo M, D’Incecco A, Cappuzzo F. Rare mutations in non-small-cell lung cancer. Future Oncol 2013;9:699-711. [PubMed]
- Landi L, Cappuzzo F. HER2 and lung cancer. Expert Rev Anticancer Ther 2013;13:1219-28. [PubMed]
- Travis WD, Brambilla E, Van Schil P, et al. Paradigm shifts in lung cancer as defined in the new IASLC/ATS/ERS lung adenocarcinoma classification. Eur Respir J 2011;38:239-43. [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [PubMed]
- Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233-41. [PubMed]
- Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol 2009;33:462-9. [PubMed]
- Watanabe S, Watanabe T, Arai K, et al. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg 2002;73:1071-5. [PubMed]
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. [PubMed]
- Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg 2001;71:971-4. [PubMed]
- Koike T, Togashi K, Shirato T, et al. Limited resection for noninvasive bronchioloalveolar carcinoma diagnosed by intraoperative pathologic examination. Ann Thorac Surg 2009;88:1106-11. [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [PubMed]
- Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 2004;28:198-206. [PubMed]
- Oka S, Hanagiri T, Uramoto H, et al. Surgical resection for patients with mucinous bronchioloalveolar carcinoma. Asian J Surg 2010;33:89-93. [PubMed]
- De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. Am J Clin Pathol 2009;131:694-700. [PubMed]
- Matsubara D, Ishikawa S, Sachiko O, et al. Co-activation of epidermal growth factor receptor and c-MET defines a distinct subset of lung adenocarcinomas. Am J Pathol 2010;177:2191-204. [PubMed]
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Prognostic significance of the proposed IASLC/ATS/ERS revised classification of lung adenocarcinoma in 514 stage I lung adenocarcinomas . Mod Pathol 2011;24:653-64. [PubMed]
- Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008;32:810-27. [PubMed]
- Kim YH, Ishii G, Goto K, et al. Dominant papillary subtype is a significant predictor of the response to gefitinib in adenocarcinoma of the lung. Clin Cancer Res 2004;10:7311-7. [PubMed]
- Shedden K, Taylor JM, Enkemann SA, et al. Gene expression-based survival prediction in lung adenocarcinoma: a multi-site, blinded validation study. Nat Med 2008;14:822-7. [PubMed]
- Sholl LM, Yeap BY, Iafrate AJ, et al. Lung adenocarcinoma with EGFR amplification has distinct clinicopathologic and molecular features in never-smokers. Cancer Res 2009;69:8341-8. [PubMed]
- Zhang J, Liang Z, Gao J, et al. Pulmonary adenocarcinoma with a micropapillary pattern: a clinicopathological, immunophenotypic and molecular analysis. Histopathology 2011;59:1204-14. [PubMed]
- Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. [PubMed]
- Maeda R, Isowa N, Onuma H, et al. Lung adenocarcinomas with micropapillary components. Gen Thorac Cardiovasc Surg 2009;57:534-9. [PubMed]
- Miyoshi T, Shirakusa T, Ishikawa Y, et al. Possible mechanism of metastasis in lung adenocarcinomas with a micropapillary pattern. Pathol Int 2005;55:419-24. [PubMed]
- Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 2002;26:358-64. [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [PubMed]
- Ohe M, Yokose T, Sakuma Y, et al. Stromal micropapillary component as a novel unfavorable prognostic factor of lung adenocarcinoma. Diagn Pathol 2012;7:3. [PubMed]
- Garfield DH, Cadranel J, West HL. Bronchioloalveolar carcinoma: the case for two diseases. Clin Lung Cancer 2008;9:24-9. [PubMed]
- Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol 2007;212:287-94. [PubMed]
- Lee HY, Lee KS, Han J, et al. Mucinous versus nonmucinous solitary pulmonary nodular bronchioloalveolar carcinoma: CT and FDG PET findings and pathologic comparisons. Lung Cancer 2009;65:170-5. [PubMed]
- Copin MC, Buisine MP, Leteurtre E, et al. Mucinous bronchioloalveolar carcinomas display a specific pattern of mucin gene expression among primary lung adenocarcinomas. Hum Pathol 2001;32:274-81. [PubMed]
- Ishida M, Igarashi T, Teramoto K, et al. Mucinous bronchioloalveolar carcinoma with K-ras mutation arising in type 1 congenital cystic adenomatoid malformation: a case report with review of the literature. Int J Clin Exp Pathol 2013;6:2597-602. [PubMed]
- Loo PS, Thomas SC, Nicolson MC, et al. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol 2010;5:442-7. [PubMed]
- Barletta JA, Perner S, Iafrate AJ, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med 2009;13:1977-86. [PubMed]
- Deshpande CG, Yoshizawa A, Motoi N, et al. Clear cell change in lung adenocarcinoma: a cytologic change rather than a histologic variant. Mod Pathol 2009;22:1595.
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [PubMed]
- Roh MS, Lee JI, Choi PJ, et al. Relationship between micropapillary component and micrometastasis in the regional lymph nodes of patients with stage I lung adenocarcinoma. Histopathology 2004;45:580-6. [PubMed]
- Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol 2004;11:297-303. [PubMed]
- Nomori H, Iwatani K, Kobayashi H, et al. Omission of mediastinal lymph node dissection in lung cancer: its techniques and diagnostic procedures. Ann Thorac Cardiovasc Surg 2006;12:83-8. [PubMed]
- Sinn HP, Helmchen B, Wittekind CH. TNM classification of breast cancer: changes and comments on the 7th edition. Pathologe 2010;31:361-6.
- Talsma K, van Hagen P, Grotenhuis BA, et al. Comparison of the 6th and 7th Editions of the UICC-AJCC TNM Classification for Esophageal Cancer. Ann Surg Oncol 2012;19:2142-8.
- Rice TW, Blackstone EH. Esophageal cancer staging: past, present, and future. Thorac Surg Clin 2013;23:461-9. [PubMed]
- Ha SY, Roh MS. The new 2011 international association for the study of lung cancer/american thoracic society/european respiratory society classification of lung adenocarcinoma in resected specimens: clinicopathologic relevance and emerging issues. Korean J Pathol 2013;47:316-25. [PubMed]
- Warth A, Stenzinger A, von Brünneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J 2012;40:1221-7. [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [PubMed]
- Sozzi G, Pastorino U, Croce CM. MicroRNAs and lung cancer: from markers to targets. Cell Cycle 2011;10:2045-6. [PubMed]
- Xie Y, Todd NW, Liu Z, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer 2010;67:170-6. [PubMed]
- Lebanony D, Benjamin H, Gilad S, et al. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol 2009;27:2030-7. [PubMed]
- Landi MT, Zhao Y, Rotunno M, et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res 2010;16:430-41. [PubMed]
- Bader AG, Brown D, Winkler M. The promise of microRNA replacement therapy. Cancer Res 2010;70:7027-30. [PubMed]
- Qi J, Mu D. MicroRNAs and lung cancers: from pathogenesis to clinical implications. Front Med 2012;6:134-55. [PubMed]
- Vaz AP, Ponnusamy MP, Batra SK. Cancer Stem Cells and Therapeutic Targets: An Emerging Field for Cancer Treatment. Drug Deliv Transl Res 2013;3:113-20. [PubMed]
- Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res 2006;66:1883-90. [PubMed]
- Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 2009;7:330-8. [PubMed]
- Sullivan JP, Spinola M, Dodge M, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res 2010;70:9937-48. [PubMed]
- Larsen JE, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med 2011;32:703-40. [PubMed]
- Luistro L, He W, Smith M, et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res 2009;69:7672-80. [PubMed]
- Wei P, Walls M, Qiu M, et al. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther 2010;9:1618-28. [PubMed]
- Nguyen NP, Almeida FS, Chi A, et al. Molecular biology of breast cancer stem cells: potential clinical applications. Cancer Treat Rev 2010;36:485-91. [PubMed]
- Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010;7:44-54. [PubMed]
- Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells 2010;28:639-48. [PubMed]
- Park HJ, Griffin RJ, Hui S, et al. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177:311-27. [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [PubMed]
- Chan OS, Yeung RM, Hung AW, et al. Stereotactic ablative radiotherapy for medically inoperable early stage lung cancer: early outcomes. Hong Kong Med J 2012;18:412-8. [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43. [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Bilal H, Mahmood S, Rajashanker B, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258-65. [PubMed]
- Pechet TT, Carr SR, Collins JE, et al. Arterial invasion predicts early mortality in stage I non-small cell lung cancer. Ann Thorac Surg 2004;78:1748-53. [PubMed]
- Yilmaz A, Duyar SS, Cakir E, et al. Clinical impact of visceral pleural, lymphovascular and perineural invasion in completely resected non-small cell lung cancer. Eur J Cardiothorac Surg 2011;40:664-70. [PubMed]
- Miyoshi K, Moriyama S, Kunitomo T, et al. Prognostic impact of intratumoral vessel invasion in completely resected pathologic stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:429-34. [PubMed]
- Mulligan CR, Meram AD, Proctor CD, et al. Lung cancer staging: a case for a new T definition. Ann Thorac Surg 2006;82:220-6. [PubMed]
- Poncelet AJ, Cornet J, Coulon C, et al. Intra-tumoral vascular or perineural invasion as prognostic factors for long-term survival in early stage non-small cell lung carcinoma. Eur J Cardiothorac Surg 2008;33:799-804. [PubMed]
- Tantraworasin A, Saeteng S, Lertprasertsuke N, et al. Prognostic factors of tumor recurrence in completely resected non-small cell lung cancer. Cancer Manag Res 2013;5:77-84. [PubMed]
- Kittaneh M, Montero AJ, Glück S. Molecular Profiling for Breast Cancer: A Comprehensive Review. Biomark Cancer 2013;5:61-70. [PubMed]
- Slodkowska EA, Ross JS. MammaPrint 70-gene signature: another milestone in personalized medical care for breast cancer patients. Expert Rev Mol Diagn 2009;9:417-22. [PubMed]
- Marisa L, de Reyniès A, Duval A, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med 2013;10:e1001453. [PubMed]
- Kratz JR, He J, Van Den Eeden SK, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet 2012;379:823-32. [PubMed]
- Jian-Wei B, Yi-Min M, Yu-Xia S, et al. Expression levels of ERCC1 and RRM1 mRNA and clinical outcome of advanced non-small cell lung cancer. Pak J Med Sci 2013;29:1158-61. [PubMed]
- Bergot E, Levallet G, Campbell K, et al. Predictive biomarkers in patients with resected non-small cell lung cancer treated with perioperative chemotherapy. Eur Respir Rev 2013;22:565-76. [PubMed]
- Roth JA, Carlson JJ. Prognostic role of ERCC1 in advanced non-small-cell lung cancer: a systematic review and meta-analysis. Clin Lung Cancer 2011;12:393-401. [PubMed]


