
Respiratory management of acute exacerbation of interstitial pneumonia using high-flow nasal cannula oxygen therapy: a single center cohort study
Introduction
Acute exacerbation of interstitial pneumonia (AE-IP), defined as an acute worsening of dyspnea with an unidentifiable cause, is a relatively common and highly morbid clinical event in patients with interstitial pneumonia (IP) (1). It was originally reported in idiopathic pulmonary fibrosis (IPF), and similar acute exacerbations were also recognized in idiopathic interstitial pneumonias (IIPs) other than IPF and interstitial pneumonia associated with collagen tissue disease (CTD-IP) (2). AE-IP patients often experience gas exchange abnormalities resulting in significant hypoxemia that requires respiratory support. Accordingly, the strategy for respiratory management of AE-IP is a matter of concern. From past studies, the benefits of invasive mechanical ventilation (IMV) in AE-IP is questionable because IMV did not improve outcomes; a systematic review of IPF patients requiring IMV for acute respiratory failure (ARF) showed a high mortality rate (3). As for noninvasive ventilation (NIV), several small retrospective studies suggested its benefits in AE-IP in the last decade; Tomii et al. showed an improved 60-day survival rate in AE-IP patients, from 27% before NIV introduction to 65% afterwards (4); and Yokoyama et al. demonstrated that early NIV treatment within 24 hours from admission was a significant predictor of survival at 30 days in AE-IP patients (5). Meanwhile, NIV failure was also reported to occur in 45–55% of IP patients with ARF, and mortality among patients receiving NIV remained high (6). Thus, the strategy for respiratory management of AE-IP has long been a controversial issue.
Recently, high-flow nasal cannula oxygen therapy (HFNC), which delivers a high flow of blended humidified oxygen through a nasal cannula, is a promising tool for respiratory support in the acute care setting (7). In a recent Japanese multicenter survey on clinical practice of HFNC reported that HFNC was used most frequently for ARF patients with IP, however, evidence for its efficacy in AE-IP is extremely scarce (8,9). Although AE-IP patients were not included in past trials investigating the efficacy of HFNC in ARF, we could speculate some concerns from a landmark RCT, the FLORALI study, which demonstrated that HFNC resulted in lower mortality rates compared with standard oxygen therapy or NIV in ARF patients (10). In fact, the majority of that study population met the recent criteria for ARDS (11), which presents pathologically with diffuse alveolar damage (DAD) similar to that of AE-IP (12). Additionally, one quarter of the study population was immunocompromised, which is also common in the AE-IP patients who are often treated with immunosuppressive agents. With the efficacy of HFNC demonstrated in the FLORALI study, it was reasonable to explore the benefits of HFNC in AE-IP.
The aim of the study is to investigate the efficacy and safety of the strategy of respiratory management of AE-IP using HFNC.
Methods
Patients
We retrospectively reviewed data on AE-IP patients admitted to Kobe City Medical Center General Hospital, Kobe, Japan, a 690-bed tertiary referral center, from June 2009 to May 2015. Since June 2012, our hospital introduced HFNC as respiratory support for patients with ARF, including AE-IP. This historical control study compared the AE-IP cohorts before and after the introduction of HFNC; pre-HFNC cohort (June 2009 – May 2012) and post-HFNC cohort (June 2012 – May 2015).
Definition of IP
We diagnosed IPF retrospectively by reviewing medical charts, high-resolution computed tomography (HRCT) images, and surgical lung biopsy patterns (when performed) based on recent guidelines in which a diagnosis of IPF without surgical lung biopsy is allowed when definitive clinical and HRCT features of IPF are present (13). Nonspecific interstitial pneumonia (NSIP) was diagnosed based on HRCT images and lung biopsy without secondary etiology. IIPs without surgical biopsy not showing definitive HRCT features of IPF (14) were collectively defined as ‘non-IPF’. Combined pulmonary fibrosis and emphysema (CPFE) was defined by the coexisting patterns of radiologic emphysematous changes and lung fibrosis without secondary etiology. Chronic hypersensitivity pneumonitis (CHP) was diagnosed based on characteristic HRCT findings, serologic and exposure history (15). CTD-IP was diagnosed when underlying collagen tissue disease was established in accordance with relevant criteria (16-19).
Definition of AE-IP
AE-IP was defined as follows according to the criteria proposed by the IPF Clinical Research Network (1), with a slight modification for IPs other than IPF: (I) a previous or concurrent diagnosis of underlying IP; (II) unexplained worsening of dyspnea within the past 30 days; (III) HRCT with new bilateral ground-glass opacity or consolidation; (IV) no evidence of pulmonary infection in bronchoalveolar lavage (BAL), endotracheal aspiration, or sputum culture, combined with negative blood tests for other potentially infectious pathogens (e.g., Pneumocystis jirovecii, Cytomegalovirus); and (V) no evidence of left heart failure, pulmonary embolism, or other possible causes of ARF.
Respiratory management of AE-IP
In both cohorts, NIV was used for first-line respiratory support in hypoxemic patients unable to maintain PaO2 >60 mmHg or SpO2 >90% on oxygen at >8 L/min using a conventional face-mask delivery system, unless they had a contraindication for NIV or had refused NIV. NIV was administered using a V60 noninvasive ventilator (Philips Respironics, Murrysville, PA, USA), with a standard reusable oronasal mask [ComfortFull (Philips Respironics) or RT040 (Fisher & Paykel Healthcare, Auckland, New Zealand)]. The initial mode for NIV was set as continuous positive airway pressure (CPAP) mode, with a positive end-expiratory pressure (PEEP) of 4–12 cmH2O. Fraction of inspired oxygen (FIO2) and PEEP were adjusted to maintain SpO2 >90%. For patients with tachypnea, accessory muscle use, or respiratory acidosis, pressure support was added with monitoring of the patients’ comfort, respiratory rate, tidal volume, and arterial blood gas data. The indication for IMV was based on the judgment of the attending physicians following a discussion of the patient’s wishes and the prognosis.
In the post-HFNC period, HFNC was also used as an alternative to NIV in accordance with the protocol of our hospital for patients (I) who had refused NIV; (II) unable to cooperate, (III) intolerant to NIV; or (IV) who improved in respiratory parameters after NIV treatment but remains unable to maintain PaO2 >60 mm Hg or SpO2 >90% on oxygen at >8 L/min using a conventional face-mask delivery system. HFNC was delivered using an Optiflow system (Fisher & Paykel Healthcare), with a total flow rate of 35–50 L/min, and the FIO2 was set to maintain SpO2 >90%.
Sedation and analgesia during respiratory support
To manage pain, agitation, and delirium in patients receiving respiratory support, we routinely used daily assessment, nonpharmacologic interventions, and as needed, analgesics and sedative agents based on the guidelines (20,21). According to pain assessment, we used intravenous fentanyl with/without nonopioid analgesics for pain and discomfort caused by endotracheal tube or noninvasive ventilation. When patients were unable to continue respiratory support due to agitation, either dexmedetomidine or propofol (propofol was used only in intubated patients) was administered and titrated to maintain light sedation between –2 to 0 on the Richmond Agitation Sedation Scale (RASS) (22). All patients were also assessed for delirium at least once a day, especially using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) in the intensive care unit (ICU) (23). For agitated patients with delirium diagnosed using CAM-ICU or by an attending physician or delirium control team based on the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) were also treated with haloperidol, atypical antipsychotic or dexmedetomidine (24). In patients with refractory dyspnea, intravenous or subcutaneous morphine with/without deep sedation using intravenous midazolam was administered for palliative treatment.
Data collection
All clinical data were obtained from medical records. The following baseline characteristics were obtained at the time of admission: age, gender, admission to ICU or intermediate care unit (IMCU), APACHE II and SAPS II score within the first 24 hours of admission, underlying IP subtype, time from first IP diagnosis to admission, underlying treatment, previous history of AE-IP, smoking history, long-term oxygen therapy, Charlson comorbidity index, vital signs, arterial blood gas data, PaO2/FIO2 ratio, lactate dehydrogenase, Krebs von der Lungen-6, and surfactant protein D. FIO2 at admission was estimated in patients receiving standard oxygen therapy as follows: (oxygen flow L/min) ×0.03+0.21 (9).
Treatments of AE-IP included pharmacotherapy such as steroids, intravenous cyclophosphamide and antibiotics, and respiratory management. In this study, we defined patients who received any respiratory support at least 24 hours as “patients treated with respiratory support”. Therefore, patients disconnected from NIV within 24 hours and then received HFNC for >24 hours were regarded as being “treated with HFNC for first-line respiratory support”. As same, patients who received any respiratory support for <24 hours and switched to standard oxygen therapy were regarded as being “treated with only standard oxygen therapy”.
Clinical outcomes measured were in-hospital mortality, 90-day mortality, adverse events, delirium, sedatives and analgesics used, and discontinuation of oral intake. To assess oral intake, we calculated the proportion of patients who discontinued oral intake for >24 hours.
Statistical analysis
Continuous variables with a normal distribution are presented as mean ± standard deviation, and variables with non-parametric distribution are presented as median (interquartile range). Categorical variables are presented as n (%). The Mann-Whitney U test was used to compare the two groups. For categorical variables, the Chi-square test was used. Survival was evaluated with Kaplan-Meier survival curves and the log rank test. Mortality at 90-day was also assessed by using a Cox-proportional hazard model with results reported as hazard ratio with 95% confidence intervals. P<0.05 were considered statistically significant. All data were analyzed by using JMP 11 (SAS Institute, Cary, NC, USA).
Results
During the entire study period, 321 consecutive IP patients were admitted to our hospital, with 96 diagnosed with AE-IP and analyzed. Fifty-three were admitted during the pre-HFNC period and 43 during the post-HFNC period.
Baseline characteristics
There were no significant differences in the baseline characteristics at admission between the two cohorts (Table 1). The proportions of patients with severe respiratory failure with PaO2/FIO2 ≤200 mmHg at admission were also similar in both cohorts (60.4% vs. 53.5%, P=0.66).
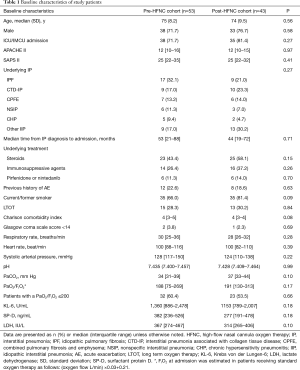
Full table
Pharmacotherapy of AE-IP
All patients were treated with high-dose corticosteroids (methylprednisolone, 250–1,000 mg/d) for 3 days followed by a tapered dosage, occasionally combined with intravenous cyclophosphamide. They also received empiric broad-spectrum antibiotic therapy until negative bacterial cultures were confirmed (Table 2).

Full table
Five patients in the post-HFNC cohort were treated with thrombomodulin and 2 of them were died in hospital. Other potential therapies for AE-IP such as polymyxin B immobilized column hemoperfusion, rituximab, plasma exchange, and intravenous immunoglobulin were not used in both cohorts. Extracorporeal membrane oxygenation and lung transplantation were not performed.
Respiratory management of AE-IP
Table 3 and Figure 1 show the respiratory support administered for AE-IP.
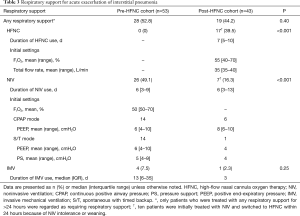
Full table
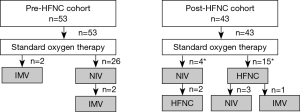
In the pre-HFNC cohort, NIV and IMV were administered to 26 (49.1%) and 4 (7.5%) patients, respectively; 25 (47.2%) patients received only standard oxygen therapy. In the post-HFNC cohort, HFNC, NIV, and IMV were administered to 17 (39.5%), 7 (16.3%), and 1 (2.3%) patient, respectively; 24 patients (55.8%) received only standard oxygen therapy.
Of the 17 patients treated with HFNC, 2 patients refused NIV; 3 patients were unable to cooperate; 4 patients were switched from NIV due to mask intolerance; and the rest of 8 patients were switched from NIV for weaning.
Survival
In the pre-HFNC cohort 26 (49.1%) patients died in hospital compared with 12 (27.9%) patients in the post-HFNC cohort (P=0.04) (Table 4). The causes of in-hospital deaths were respiratory failure in all cases. Of these, one patient died immediately following an occurrence of pneumothorax, and the rest of the patients died due to progression of AE-IP. In the subgroup of patients with a PaO2/FIO2 ≤200 mmHg, in-hospital mortality was 20 (62.5%) vs. 8 (34.8%), in pre- and post-HFNC cohorts, respectively (P=0.04). We also compared the outcomes between subgroups of patients treated with any respiratory support. In-hospital mortality was 23 (82.1%) vs. 11 (57.9%), in pre- and post-HFNC cohorts, respectively (P=0.07). Of the 17 patients treated with HFNC, 9 patients who did not receive or discontinued NIV because of refusal of NIV, inability to cooperate or NIV intolerance all died in hospital; the rest of 8 patients who were switched from NIV for weaning survived to be discharged or transferred except 1 patient.
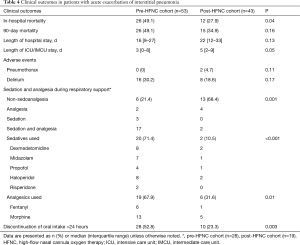
Full table
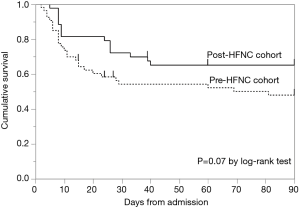
Kaplan-Meier analysis of the probability of survival from admission to day 90 showed a more favorable curve for the post-HFNC cohort, but there was no significant difference between the two cohorts (Figure 2). The hazard ratio for death at 90 days was 0.57 (95% confidence interval, 0.29 to 1.05, P=0.07) in the post-HFNC cohort compared with the pre-HFNC cohort.
Safety and QOL
Two patients developed pneumothorax during HFNC use in the post-HFNC cohort; both patients were administered NIV as first-line respiratory support followed by HFNC. The incidence of delirium was not significantly different between the two cohorts (30.2% vs. 18.6%, P=0.17). The use of sedatives and analgesics were significantly less frequent in the post-HFNC cohort (78.6% vs. 31.6%, P=0.001). The proportion of patients who discontinued oral intake for >24 hours was also significantly lower in the post-HFNC cohort (52.8% vs. 23.3%, P=0.003) (Table 4).
Discussion
To the best of our knowledge, this is the first study to investigate the effects of the respiratory management using HFNC in AE-IP patients. The present study showed that AE-IP patients had (I) lower in-hospital mortality, (II) similar incidence of complications, (III) less frequent use of sedatives and analgesics, and (IV) lower incidence of discontinuation of oral intake after the introduction of HFNC to the strategy for respiratory management of AE-IP.
Although there is a lack of evidence to support the clinical effectiveness of HFNC in AE-IP, accumulating evidence on physiological effects of HFNC indicates its possible benefits in AE-IP (9).
First, the unique mechanisms of actions of HFNC, such as effective delivery of up to 100% oxygen, washout of the pharyngeal dead space, decreased inspiratory resistance, improved secretion clearance and mucosal integrity with heated humidification, and positive end-expiratory pressure effect are potential advantages compared with standard oxygen therapy (7).
Second, lung mechanics in AE-IP and lung protective strategies are of concern. There is little evidence regarding appropriate ventilator settings for IMV and NIV in AE-IP, and expert opinions are based on the strategy of lung protective ventilation in ARDS, which includes low tidal volumes to avoid over-distention and appropriate PEEP to prevent alveolar collapse (25). However, special caution is required when ventilating AE-IP patients because they may have little recruitable lung and could be particularly prone to over-distension injury. Fernández-Pérez et al. reported that high PEEP >10 cm H2O failed to improve oxygenation in ventilated patients with IP and was associated with higher mortality (26). This result was well explained by Gattinoni et al., who showed that a higher PEEP could be harmful in ARDS patients with a low percentage of recruitable lung because it might only have overinflated lung regions that were already open (27). On the other hand, Mauri et al. revealed unique physiological effects of HFNC; they conducted a randomized cross-over study in 15 ARF patients, which showed that HFNC significantly improved oxygenation and increased end-expiratory lung volume without a change in tidal volume compared with a standard oxygen therapy (28). Braunlich also reported the effects of HFNC on ventilation in 13 IPF patients, which revealed that HFNC led to decrease in both respiratory rates and capillary PCO2 without a change in tidal volume compared with spontaneous breathing (29). Thus, HFNC might improve the respiratory parameters in a lung protective manner for AE-IP patients.
Third, patients’ comfort and compliance are also important in successful respiratory management. Previous studies reported that NIV intolerance occurred at the rate of 11.4–15% in ARF patients and was associated with intubation and mortality (30). Although data on the actual prevalence of HFNC intolerance are lacking, HFNC was reported to be better tolerated and more comfortable than standard oxygen therapy and NIV in ARF (7). In our study, of 17 patients who received HFNC, 12 patients were switched from NIV due to mask intolerance or for weaning and HFNC was well tolerated in all of those patients. Thus, HFNC could have improved patient comfort and tolerance of respiratory support compared to conventional therapy and acted as salvage therapy for NIV-intolerant patients who had no choice other than standard oxygen therapy or IMV before the introduction of HFNC. Importantly, almost all the patients who were switched from NIV to HFNC for weaning survived to be discharged or transferred, however, all the other patients who received HFNC because of NIV refusal, inability to cooperate or NIV intolerance died during hospitalization. With regards to the differences in survival between the two subgroups of the patients treated with HFNC, those who improved in respiratory parameters after NIV treatment may be potential responders to HFNC. The efficacy of HFNC for weaning from NIV in AE-IP should be determined in future studies.
It is also worthy of special mention that both the use of sedatives and analgesics and the number of patients who discontinued oral intake were decreased after the introduction of HFNC. NIV often requires sedative agents due to mask intolerance or inability to cooperate in ARF (31), however, better comfort and tolerability of HFNC might lead to less requirement of sedative agents compared to NIV. In addition, NIV masks or conventional oxygen masks usually limit oral intake during therapy, especially during severe hypoxemia, whereas interfaces of HFNC do not restrict oral intake. The incidence of delirium during HFNC use was also first reported in this study. Delirium is an important event in critically ill patients receiving respiratory support, which directly relates to survival outcomes. In the present study, the incidence of delirium was 30.2% in the pre-HFNC cohort, which is consistent with a previous report on ARF patient receiving NIV (32). Although not statistically significant, the incidence of delirium was as low as 18.6% after the introduction of HFNC. Future studies are needed to determine the effects of HFNC on delirium.
This study had several limitations. First, because this was a retrospective historical control study, there were possible confounding factors. Although baseline characteristics and major pharmacotherapy of AE-IP were not significantly different between the two cohorts, the outcomes may be biased by the severity of respiratory failure, the category of baseline IP (IPF or not). An accumulation of expertise and knowledge in managing AE-IP including sedoanalgesia during the study period could have also affected the outcomes. Second, the present study did not investigate directly whether NIV or HFNC is the better means of respiratory support for AE-IP and our results should not be overinterpreted. Caution is needed when applying HFNC in the patients who refuse NIV, unable to cooperate, or intolerant to NIV. Finally, the small number of patients studied limits the reliability of our results.
Conclusions
In conclusion, HFNC could be a feasible option in respiratory management of AE-IP. Further prospective studies are required to assess the clinical benefits of HFNC in AE-IP.
Acknowledgements
The authors would like to thank all medical staff members who participated in the management of patients in clinical practice.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Kobe City Medical Center General Hospital (approval number: zn151209).
References
- Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636-43. [Crossref] [PubMed]
- Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest 2007;132:214-20. [Crossref] [PubMed]
- Mallick S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir Med 2008;102:1355-9. [Crossref] [PubMed]
- Tomii K, Tachikawa R, Chin K, et al. Role of non-invasive ventilation in managing life-threatening acute exacerbation of interstitial pneumonia. Intern Med 2010;49:1341-7. [Crossref] [PubMed]
- Yokoyama T, Tsushima K, Yamamoto H, et al. Potential benefits of early continuous positive pressure ventilation in patients with rapidly progressive interstitial pneumonia. Respirology 2012;17:315-21. [Crossref] [PubMed]
- Vianello A, Arcaro G, Battistella L, et al. Noninvasive ventilation in the event of acute respiratory failure in patients with idiopathic pulmonary fibrosis. J Crit Care 2014;29:562-7. [Crossref] [PubMed]
- Roca O, Hernandez G, Diaz-Lobato S, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care 2016;20:109. [Crossref] [PubMed]
- Ito J, Nagata K, Sato S, et al. The clinical practice of high-flow nasal cannula oxygen therapy in adults: A Japanese cross-sectional multicenter survey. Respir Investig 2018;56:249-57. [Crossref] [PubMed]
- Lee CC, Mankodi D, Shaharyar S, et al. High flow nasal cannula versus conventional oxygen therapy and non-invasive ventilation in adults with acute hypoxemic respiratory failure: A systematic review. Respir Med 2016;121:100-8. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143-50. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315-24. [Crossref] [PubMed]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581-90. [Crossref] [PubMed]
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271-7. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [Crossref] [PubMed]
- Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119-41. [Crossref] [PubMed]
- Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263-306. [Crossref] [PubMed]
- Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338-44. [Crossref] [PubMed]
- Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001;29:1370-9. [Crossref] [PubMed]
- American Psychiatric Association, American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed. Washington, DC: American Psychiatric Association; 1994. xxvii, p. p 886.
- Papiris SA, Manali ED, Kolilekas L, et al. Clinical review: idiopathic pulmonary fibrosis acute exacerbations--unravelling Ariadne's thread. Crit Care 2010;14:246. [Crossref] [PubMed]
- Fernández-Pérez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest 2008;133:1113-9. [Crossref] [PubMed]
- Gattinoni L, Caironi P, Cressoni M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 2006;354:1775-86. [Crossref] [PubMed]
- Mauri T, Turrini C, Eronia N, et al. Physiologic Effects of High-flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am J Respir Crit Care Med 2017;195:1207-15. [Crossref] [PubMed]
- Bräunlich J, Beyer D, Mai D, et al. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration 2013;85:319-25. [Crossref] [PubMed]
- Kwok H, McCormack J, Cece R, et al. Controlled trial of oronasal versus nasal mask ventilation in the treatment of acute respiratory failure. Crit Care Med 2003;31:468-73. [Crossref] [PubMed]
- Devlin JW, Nava S, Fong JJ, et al. Survey of sedation practices during noninvasive positive-pressure ventilation to treat acute respiratory failure. Crit Care Med 2007;35:2298-302. [Crossref] [PubMed]
- Charlesworth M, Elliott MW, Holmes JD. Noninvasive positive pressure ventilation for acute respiratory failure in delirious patients: understudied, underreported, or underappreciated? A systematic review and meta-analysis. Lung 2012;190:597-603. [Crossref] [PubMed]


