
Radical aggressive treatment among non-small cell lung cancer patients with malignant pleural effusion without extra-thoracic disease
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, approximately 85% of all lung cancer cases are non-small cell lung cancer (NSCLC) (1,2). Despite advances in early detection and its proved impact on treatment success, the majority of patients are diagnosed with advanced stage disease. The therapeutic options for patients with advanced NSCLC are limited, which consequently entails a poor prognosis (3).
Malignant pleural effusion (MPE) is defined as the accumulation of significant amount of pleural exudate, accompanied by the presence of malignant tumor cells in the pleural space, the pleural fluid or the parietal pleura (4,5). Lung cancer is the most frequent cause of MPE, accounting for approximately 40% of all cases. In the 7th edition of the Tumor Node Metastasis classification the presence of MPE is considered as an M1a descriptor (stage IV). However, although MPE is a common indicator of highly metastatic disease, it can also occur in patients without extra-thoracic disease (6).
The treatment of patients with MPE without extra-thoracic lesions is the same as that for patients with distant metastases, usually involving palliative chemotherapy without surgical intervention (7,8).
Recent reports have underscored the benefit of a surgical approach for the treatment of patients with carcinomatous pleuritic and minimal disease. These reports showed that performing an elective extrapleural pneumonectomy (EPP) improves overall survival (OS), with a 5-year survival rate ranging from 22% to 33.7% (9,10).
However, there is still limited information about the effect of aggressive treatment. In this article, we present our institutional experience using aggressive surgical management in M1a NSCLC patients with MPE without extra-thoracic disease.
Material and methods
Selection of the patients
A retrospective study was conducted in patients with advanced NSCLC adenocarcinoma with MPE treated at the Thoracic Oncology Unit of the Instituto Nacional de Cancerología in Mexico City, from 2012 to 2016. The patients were presented, selected, and their therapeutic plan was discussed in the clinical sessions of the Functional Thoracic Unit. The protocol was approved by the Scientific and Bioethical Committee of the Instituto Nacional de Cancerología, register CEI/957. All patients signed informed consent forms prior to enrollment in the study. Six NSCLC adenocarcinoma patients with MPE, stage M1a without extra-thoracic disease, with diagnosis confirmed by histology, PET-CT and MRI were included. Patients received 3–6 cycles of systemic therapy before surgical treatment [either with platinum-based chemotherapy or tyrosine kinase inhibitor (TKI), according to genotyping status] according to National Comprehensive Cancer Network (NCNN) recommendations, subsequently, underwent lobectomy plus decortication or EPP. Clinical variables, mutational status [EGFR, KRAS, ALK, or wild type (WT)], and treatment for the primary tumor data were collected for analysis. Progression-free survival (PFS) and OS were measured from the time of diagnosis. The better response to first-line treatment was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.1.
Determination of EGFR and KRAS mutational status
Biopsies were taken using CT-guided Tru-cut or by bronchoscopy, genomic DNA was extracted from the areas of paraffin slides by a standard procedure using QIAamp DNAFFPE tissue kit (QIAGEN), according to the instructions provided by the manufacturer. EGFR [exon 19, exon 18 (G719X), exon 20 (T790M and S768I), exon 21 (L858R and L861Q)] and KRAS (codon 12 12ALA, 12ASP, 12ARG, 12CYS, 12SER, 12VAL, and codon 13 13ASP) gene mutations were detected by Therascreen RGQ PCR kit (QIAGEN, Scorpions ARMS method). ALK rearrangements were identified by fluorescence in situ hybridization [Vysis LSI ALK (2p23) Dual Color, Break Apart Rearrangement Probe, Abbott Molecular]. Additionally, the biopsies of two patients were evaluated by next generation sequencing (NGS) using Illumina platform.
Pulmonary rehabilitation
The patients were trained by a physiotherapist to master adequate breathing and coughing techniques, instructed on incentive respiratory exercise, in addition, exercises for chest expansion and shoulder girdle mobilization were executed. Spirometry, 6-minute walking test and evaluation of symptomatic status were performed at the beginning and the end of pulmonary rehabilitation.
Radiotherapy
The patients were treated using a linear accelerator Varian, Unique and TrueBeam, with energy 6 MV; RapidArc IMRT technique was used with a dose prescription of 60 Gy in 30 fractions directed to the surgical field and 54 Gy in 27 fractions directed to mediastinal nodes if the nodes were positives after the surgery.
Statistical analysis
Continuous variables were summarized as arithmetic means, and standard deviations. Categorical variables were summarized as frequencies and percentages. PFS to first-line treatment was estimated from the date of treatment begin until disease progression or last follow-up. OS was measured from the day at diagnosis to day of death or last follow-up visit. PFS and OS times were calculated by means of Kaplan-Meier method. Statistical significance was determined as P<0.05 using a two-tailed test. SPSS software version 20 (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis.
Results
A total of six patients were included in the study. Table 1 summarizes the relevant clinical variables among the study population. All patients had a M1a NSCLC adenocarcinoma, four patients were female and 2 were male. Age ranged from 33 to 67 years. All had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1. Most patients (83%) had tobacco exposure. Two patients harbored EGFR mutations, one patient had PI3K/BRAF mutation, and another had HER2 mutation.
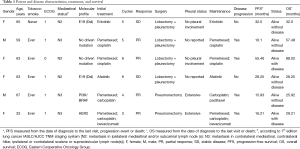
Full table
All the patients received systemic treatment, four received chemotherapy and two received TKIs therapy. As best response, two patients achieved stable disease, and 4 reached partial response (PR). The median time to surgery was 4.8 months (ranging from 3 to 6 months). Four patients underwent lobectomy plus pleurectomy and two underwent pneumonectomy. The pathology report showed two patients with extensive pleural invasion, two patients without pleural invasion, and the rest of the reports, the pleural status was not specified. All patients received radiotherapy to the chest wall, if they had mediastinal node positives, received treatment to the affected regions. The median PFS was 15.9 months (95% CI: 15.6–55.5 months). At the time of analysis, two patients had not progressed to radical treatment. After recurrence, the patients received local-ablative therapy. The median OS was not reached. All patients were alive at the time of the analysis, 4 without evidence of disease.
Clinical cases
Case 1
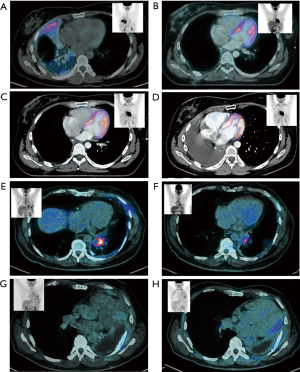
A 67-year-old male with smoking history, in January 2016 the patient reported chest pain, and in April 2016 cough and dyspnea with nocturnal exacerbation were described. A chest X-ray was performed diagnosing left pleural effusion. CT detected tumoral activity in left pleura, pleural effusion and left parahiliary nodules of juxta-fissural location. No intracranial lesions were detected using MRI. A PET-CT study confirmed exclusively intrathoracic affection localized in left lung, thickening of the parietal and mediastinal pleura, mediastinal lymph nodes at 4, 5, 7, 10L, 10R levels; multiple nodes distributed randomly in the parenchyma of both lungs. Basal carcinoembryonic antigen (CEA) level was 173 ng/mL (Figure 1A,B,C,D).
Left thoracoscopy plus wedge biopsy, left inferior lobe and parietal pleural biopsy were performed. The histopathological results reported moderately differentiated adenocarcinoma with predominantly acinar (90%) and micropapillary (10%) pattern, nodule size greater than 0.7 cm × 0.4 cm × 0.5 cm, with multiple subpleural nodules and in contact with the surgical resection border.
Systemic therapy was prescribed with carboplatin and pemetrexed for 6 cycles reaching a RP with a reduction in tumoral volume of 60%, thereafter, surgical approach was decided to improve local control of the disease after discussing the clinical case in the Functional Thoracic Unit.
In October 2016, a left posterolateral thoracotomy with pleuropneumonectomy, partial pericardiectomy and mediastinal dissection was performed. Pathological results reported the presence of moderately differentiated, multifocal adenocarcinoma with predominantly cribriform pattern (60%), acinar (20%), papillary (15%) and lepidic (5%) with secondary changes to treatment 15%, plus vascular-lymphatic permeation, neural invasion and infiltration to the visceral pleura. Nodal involvement was described in 1 of 3 lymph nodes. Pericardial tissue and pleural samples with metastasis of adenocarcinoma. NGS exhibited PI3K and BRAF (V600E) mutations. After surgical procedure, a PET-CT described post-surgical alteration with no suggestive data of tumoral hypermetabolism. Adjuvant chest wall external beam radiotherapy (EBRT) was administered, 60 Gy in 30 fractions to the surgical field and 54 Gy in 27 fractions to the mediastinum. The Thoracic Unit decided to continue with systemic treatment with carboplatin and paclitaxel for 6 cycles, achieving complete response by PET-CT.
Case 2
A 33-year-old female with no comorbidity history. In July 2015, presented progressive dyspnea and dry cough. In November 2015 developed right abdominal pain and increased dyspnea. The CT scan detected right diffuse infiltration and the cytology of the pleural effusion demonstrated the presence of adenocarcinoma. Basal CEA level was 5.11 ng/mL. A PET-CT study confirmed exclusively intrathoracic affection. In December 2015, underwent to right thoracoscopy, with histopathological report of moderately differentiated adenocarcinoma with lepidic (80%) and micropapillary (20%) pattern; a mutation profile of CK 7 (+), TTF-1 (+), ACE (+) and EGFR (−) was reported.
The patient received systemic therapy with carboplatin, pemetrexed and bevacizumab completing 3 cycles in February 2016, reaching stable disease, nevertheless, the respiratory symptoms worsened, deteriorating the quality of life. In March 2016 it was decided to perform surgical treatment, with right thoracotomy and pneumonectomy. Pathological results reported the presence of moderately differentiated adenocarcinoma, with lepidic pattern (95%) and bronchial positive margin, one mediastinal adenopathy with carcinoma. NGS reported HER2 amplification. In April 2016, the patient restarted systemic therapy with carboplatin, pemetrexed and bevacizumab, completing 6 cycles, referring improvement in quality of life. In Jun 2016 adjuvant chest wall EBRT was administered, 60 Gy in 27 fractions to the surgical field, followed by mediastinal IMRT 54 Gy in 27 fractions to the mediastinum with concomitant carboplatin. In April 2017 the patient presented mediastinic progression and received systemic treatment with pemetrexed and carboplatin, at the time of the analysis remained alive and receiving treatment (Figure 1E,F,G,H).
Discussion
The prognosis for patients with lung cancer remains dismal, with a 5-year OS rate of 16%. Patients with advanced-stage disease (IIIB and IV) have a particularly low survival and a minimal chance to be cured (3). Surgery is the main therapeutic option for patients with early stage NSCLC. In the metastatic setting, specific surgical techniques, such as video-assisted thoracoscopy, can be helpful for the management of pleural effusions or local complications (4). However, the efficacy of curative surgical treatment in combinations with systemic treatments (out of palliative procedures) in patients with advanced NSCLC remains controversial (7).
EPP is a radical surgical procedure that is commonly performed for the treatment of malignant pleural mesothelioma. Nevertheless, this procedure has also been used in the treatment of MPE, with encouraging results when performed in selected patients after induction chemotherapy (10-12). In a retrospective study performed in Japan, 152 patients with NSCLC and pleural carcinomatosis (without extrathoracic disease), underwent macroscopic pleural complete resection by EPP, reaching 5-year OS rates of 37.1% (13). MPE can be managed with thoracotomy and decortication, this is generally recommended for patients with severe symptoms for whom other interventions have failed (4). Furthermore, it has been reported that pleurectomy decreases the local recurrence rate in patients with MPE (14).
No randomized trials reporting the effectiveness of pleurectomy or decortication for MPEs have been performed. Furthermore, decortication has generally been restricted to patients with significant symptoms and prolonged life expectancy for whom other therapeutic interventions have failed (4).
In this study, we present the cases of six patients with MPE secondary to NSCLC without extra-thoracic disease and treated with systemic therapy. These patients received aggressive surgical treatment and radiotherapy to the chest wall. In these patients, the surgical treatment clearly offered better symptom control while increasing the effectiveness of systemic therapy. These results are in agreement with a previous study showing that the postoperative prognosis of patients with MPE after thoracotomy is reasonably favorable (7,15).
In this study, we show that disease control was adequate following aggressive surgical therapy, improving both PFS and OS compared with conservative treatment, regardless of the mutational profile (EGFR or ALK mutation), PD-L1 expression, or type of surgical treatment (pneumonectomy or lobectomy plus pleurectomy).
No surgical complications were found, and in agreement with previous reports, macroscopic complete resection was associated with better survival (13). At the last follow-up, all patients were alive, 4 without macroscopic tumoral activity and 2 of them without disease progression after radical treatment.
This study has some limitations, it is a retrospective study with a small sample, and the surgeries were performed at a reference hospital. A randomized, prospective study with a bigger sample is needed to confirm these results.
Conclusions
Patients with MPE secondary to NSCLC without extra-thoracic disease may be candidates for aggressive surgical treatment, nevertheless, it is necessary to conduct randomized, prospective studies to evaluate this therapeutic option in highly specialized centers with experience in these procedures. The aggressive treatment in M1a NSCLC patients could improve OS and PFS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by the Scientific and Bioethical Committee of the Instituto Nacional de Cancerología, register CEI/957. All patients signed informed consent forms prior to enrollment in the study.
References
- Pilleron S, Sarfati D, Janssen-Heijnen M, et al. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J Cancer 2019;144:49-58. [Crossref] [PubMed]
- Ortega-Gómez A, Rangel-Escareño C, Molina-Romero C, et al. Gene-expression profiles in lung adenocarcinomas related to chronic wood smoke or tobacco exposure. Respir Res 2016;17:42. [Crossref] [PubMed]
- Raez LE, Santos ES, Rolfo C, et al. Challenges in Facing the Lung Cancer Epidemic and Treating Advanced Disease in Latin America. Clin Lung Cancer 2017;18:e71-9. [Crossref] [PubMed]
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013;34:459-71. [Crossref] [PubMed]
- Morgensztern D, Waqar S, Subramanian J, et al. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol 2012;7:1485-9. [Crossref] [PubMed]
- Kasapoglu US, Arınç S, Gungor S, et al. Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J 2016;10:791-9. [Crossref] [PubMed]
- Ren Y, Dai C, Shen J, et al. The prognosis after contraindicated surgery of NSCLC patients with malignant pleural effusion (M1a) may be better than expected. Oncotarget 2016;7:26856-65. [Crossref] [PubMed]
- Bonomi M, De Filippis C, Lopci E, et al. Clinical staging of malignant pleural mesothelioma: current perspectives. Lung Cancer (Auckl) 2017;8:127-39. [Crossref] [PubMed]
- Johnson KK, Rosen JE, Salazar MC, et al. Outcomes of a Highly Selective Surgical Approach to Oligometastatic Lung Cancer. Ann Thorac Surg 2016;102:1166-71. [Crossref] [PubMed]
- Fukui T, Yokoi K. The role of surgical intervention in lung cancer with carcinomatous pleuritis. J Thorac Dis 2016;8:S901-7. [Crossref] [PubMed]
- Yamaguchi M, Ichinose Y, Shimamatsu S, et al. Preoperative concurrent chemoradiotherapy followed by extrapleural pneumonectomy for patients with non-small cell lung cancer with malignant pleural effusion and/or pleural nodules: Ten-year results of a prematurely terminated single institute phase II trial. Surg Oncol 2015;24:78-83. [Crossref] [PubMed]
- Wolf AS, Flores RM. Extrapleural pneumonectomy for pleural malignancies. Thorac Surg Clin 2014;24:471-5. [Crossref] [PubMed]
- Iida T, Shiba M, Yoshino I, et al. Surgical Intervention for Non-Small-Cell Lung Cancer Patients with Pleural Carcinomatosis: Results From the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 2015;10:1076-82. [Crossref] [PubMed]
- Ren YJ, She YL, Dai CY, et al. Primary tumour resection showed survival benefits for non-small-cell lung cancers with unexpected malignant pleural dissemination. Interact Cardiovasc Thorac Surg 2016;22:321-6. [Crossref] [PubMed]
- Zamboni MM, da Silva CT Jr, Baretta R, et al. Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med 2015;15:29. [Crossref] [PubMed]


