
Barriers to conducting a multi-center randomized controlled trial of surgical stabilization of rib fractures (and how to overcome them)
The elusive multicenter randomized controlled trial (RCT) represents the “holy grail” of research addressing the efficacy of surgical stabilization of rib fractures (SSRF). The practice of SSRF began as a last ditch effort to salvage patients with the most severe form of chest wall injury: flail chest. Cases were performed sporadically, in a non-standardized fashion, and using equipment intended for facial bones (1). Over the last 40 years, SSRF has evolved into a standardized operation performed routinely at many trauma centers. The exponential growth of SSRF nationally over the last 10 years was quantified recently in our review of the National Trauma Database (2). Furthermore, penetrance of the operation into academia is underscored by the Eastern Association for the Surgery of Trauma’s recent clinical practice guideline advocating for consideration of SSRF in all patients with flail chest (3), as well as the American Association for the Surgery of Trauma’s updated fellowship case requirements, which now include SSRF as a recommended operation (4). Finally, SSRF-specific Common Procedural Terminology codes have come into existence within the last 5 years.
Despite the increased visibility of SSRF, it remains a highly controversial operation. Areas of ongoing controversy include influence of (and conflicts of interest with) industry (5), choice of fixation platform, and establishment of competency standards to perform the operation. However, the most commonly provided reason to question SSRF remains a lack of compelling data to support its efficacy, specifically in the form of a multicenter RCT. Mayberry et al. surveyed trauma, orthopedic, and thoracic surgeons regarding their opinions of SSRF (6). Approximately one quarter of respondents indicated that the operation was rarely, if ever, indicated; 90% of these opponents indicated that a multicenter RCT would be necessary to change their negative opinion.
The call for a definitive RCT has been echoed by many surgeons since the aforementioned publication, as well as intensified by recent data documenting the rapid growth of SSRF at lower trauma level designation hospitals in patients without flail chest (2). Why then, has this task not been accomplished? This chapter will review the theoretical obstacles involved in the design and execution of a multi-center RCT of SSRF, as well as provide guidance to overcoming these obstacles.
Disclaimer
In March, 2017, the Chest Wall Injury Society (CWIS) began planning for CWIS NON-FLAIL, a multicenter RCT of SSRF in patients with severe, non-flail fracture patterns (NCT03221595, www.cwisociety.org/research/nonflailrct). As of this writing (December, 2018) the trial has enrolled 48/100 subjects from ten American, academic medical centers. The observations made herein are based in large part on the experiences of the authors surrounding the design and execution of that trial.
Do we really need a multicenter RCT of SSRF?
Not every disease requires a RCT to inform treatment. Indeed, in some cases, allocating the resources necessary to conduct a proper RCT would be impractical, wasteful, and even dangerous. This notion was famously parodied in the article of Smith and Pell, who sarcastically lamented the lack of RCTs addressing the efficacy of a parachute to prevent death from “gravitational challenge” (7). The authors specifically criticized the overly rigorous approach of advocates of evidence-based medicine, who would refuse to adopt all interventions in the absence of a RCT.
The efficacy of SSRF differs obviously to that of a parachute; however, it is a helpful exercise to consider the reasons why. First, there remains relative equipoise for operative, as compared to non-operative management of patients with severe chest wall injuries. Clinical equipoise refers to genuine uncertainty over whether SSRF will be beneficial, which then forms the ethical basis for assigning subjects to different treatment arms of a trial. Equipoise regarding SSRF appears to be present within the medical community as evidenced by both surveys (6,8) and consensus statements (3,9). Second, SSRF is not currently the universal, standard of care at most hospitals. Whereas (almost) no one would currently consider jumping from an airplane without a parachute—the majority of trauma centers do not offer SSRF. Finally, the costs associated with routine adoption of SSRF are both great and complex. Although the surgery may ultimately decrease healthcare costs, the person power required to disseminate the technique and equipment is not insignificant.
Having established that the efficacy of SSRF should be studied, the next issue involves the level of evidence necessary to inform recommendations: is the current literature enough to answer the question definitively without expending the resources necessary for a multicenter RCT? The certainty of medical evidence is most commonly quantified using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology (Table 1) (10). This system consists of levels of data, on which grades of recommendations are based. The highest grade of recommendation (Grade A) is based upon the highest level of evidence (Level 1), which requires either systematic review with homogeneity of RCTs (level 1a), or individual RCTs with a narrow confidence interval (level 1b). The medical community has thus agreed upon the RCT as the standard study design against which all others are compared.

Full table
There is no shortage of published studies (including RCTs) addressing the efficacy of SSRF. However, the majority of these studies are retrospective in nature and, specifically, case control studies. Epidemiologically, case control studies are unable to prove causation; rather, they can only document an association between a predictor variable (e.g., SSRF) and an outcome variable (e.g., pneumonia). The associations identified in retrospective case control studies, however, are frequently limited by both incomplete/inaccurate data and bias. Parameters abstracted in a retrospective fashion are by definition fixed as the data have already been collected. Thus, the researcher must mold his or her research question to fit the available information. This strategy can unfortunately result in “reverse research” or “data mining,” in which retrospectively collected data are scanned for an association, and a hypothesis is then generated retroactively and based upon data availability. Finally, retrospective analyses of SSRF are typically limited to broad, generic outcomes such as mortality or length of stay, given that these are the fields most readily available in both institutional and national datasets. The ability to construct standardized variables a priori (e.g., quality of life or narcotic requirements) is only possible in prospective studies.
Bias is defined as any systematic error in the design, conduct, or analysis of a study that results in a mistaken estimate of the exposure’s effect on the outcome. In the SSRF literature, selection bias is the most common factor that blurs meaningful conclusions. Selection bias may occur in any investigation in which subjects are not randomized to treatment, including both retrospective and prospective studies. This bias occurs in two broad directions in the SSRF literature. The first scenario involves the common finding that patients who undergo SSRF are, in general, less severely injured as compared to those who do not. Favorable outcomes observed in patients who undergo SSRF, as compared to those managed non-operatively, may thus be due to overall prognosis rather than any potential benefit of the surgery. Selection bias may also confounded the relationship between timing of surgery and outcomes among patients who undergo SSRF. Specifically, patients selected for early surgery may be systematically less injured than those who do not (consider the patient with an intra-cranial pressure monitor, open abdomen and rib fractures, as compared to the same rib fractures in a patient without additional injuries) (11).
Interestingly, selection bias may also operate in the reverse direction among patients with isolated chest wall injuries. Specifically, patients with the most severe rib fractures, in whom most surgeons agree that there is benefit to surgery, would be most likely to receive the operation. Patients with less severe injuries (e.g., three bicortically displaced fractures), by contrast, may not be offered surgery. In this case, improved outcomes in the latter patient group are likely due to less severe injuries rather than a non-operative treatment strategy. One final type of bias that is frequently of concern in the SSRF literature involves studies with a cross over design. For example, our prospective trial of SSRF compared all patients managed non-operatively over a 1-year period to all patients managed operatively over the subsequent year (12). The concern here is that additional practice changes may have occurred over the time period of the study that would have selectively benefited the operative patients. Similarly, pooling non-standardized data from multiple institutions or surgeons (even if over similar time periods) introduces variability in practice patterns. Although various statistical tests are available to mitigate the effects of bias, including subgroup and regression analyses, these maneuvers require that any potentially confounding variables are both recognized and abstracted: the potential for unmeasured bias, however, always remains.
To date, three RCTs of SSRF have been published (Table 2), each of which has been limited to patients with flail chest, and each of which demonstrated benefit to surgery in the acute period in terms of decreased incidence and duration of mechanical ventilation. Additional outcomes, including pneumonia, quality of life, mortality, and pulmonary function, were variable. Although each of these trials represent tremendous contributions to the literature on SSRF, important limitations remain. Many advancements in the peri-operative management of patients with severe chest wall injuries have occurred since their publication. Loco-regional and system analgesic regimens, operative technique and fixation method, and time from injury to surgery represent examples of such advancements. The SSRF operation performed by Tanaka in 2002 (13) is likely vastly different from the one performed by many surgeons currently (16). Furthermore, certain daily inpatient parameters, such as narcotic requirements and spirometry, were not routinely abstracted. Next, these three trials represent relatively small samples of patients with a specific type of chest wall injury (flail chest) from single institutions and, in some cases, single surgeons. Finally, although the overall conclusions drawn from the three trials were in favor of SSRF, there was not a uniform benefit observed in either outpatient quality of life or pulmonary function.

Full table
The results of the three published RCTs, as well as other studies, have been grouped into multiple meta-analyses (17-20). Meta-analysis involves the pooling of multiple individual studies to increase power, and thus minimize type II error. Meta-analysis is also able to assess for homogeneity of trials and inform generalizability. However, important limitations of meta-analyses include variable quality of individual studies, lack of uniform definitions of both independent and dependent variables, and publication bias. Each of the four meta-analyses of the SSRF literature appropriately recognized their limitations.
In conclusion, the current literature pertaining to the efficacy of SSRF is in general positive. However, deficiencies in both the quantity and quality of studies, sometimes conflicting results, and dated RCTs, coupled with the exponential increase in the practice of the operation, reinforce the premise of persistent clinical equipoise and justify expending the resources necessary to conduct a contemporary multicenter RCT.
Specific barriers (and how to overcome them)
Defining the research question
The first barrier to conducting the “definitive” multicenter RCT of SSRF is that such a trial is theoretically impossible to perform. No one trial, regardless of either sample size or patient population, will unequivocally answer the question “is SSRF effective?” because the research question is too general. Rib fractures represent a tremendously diverse injury pattern in both clinical and radiographic scope. Both patient demographics and associated injuries add additional layers to the puzzle. Previous investigators have attempted to narrow the research question by limiting inclusion to a specific injury pattern, most commonly flail chest. However, the diagnosis of “flail chest” is fraught with ambiguity and, accordingly, many surgeons have currently expanded indications for SSRF well beyond the traditional, clinical diagnosis of flail chest. Investigations of these “non-flail” patients, however, have remained markedly limited by both radiographic and clinical variability.
To be relevant, a RCT of SSRF should target a specific patient population, a specific fracture pattern, and a specific set of outcomes. To accomplish this task, a balance must be struck between being too general, in which case the results of the trial are not applicable to any one patient, and being too specific, in which case the study will only apply to the minority of rib fracture patients (and probably not accrue enough patients to be completed). Accepting that several RCTs will be necessary to answer a group of questions regarding the efficacy of SSRF is the first step towards making progress in this field of research.
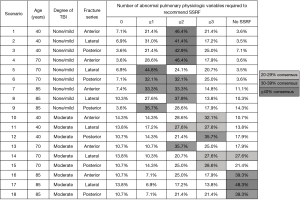
The Chest Wall Injury Society attempted to define a research question for which there was clinical equipoise by conducting a survey of its members (8). In the survey, a baseline patient with ≥3 bicortically displaced fractures, no clinical or radiographic evidence of flail chest, and no competing operative injuries was presented. Next, patient age, pulmonary derangement, degree of traumatic brain injury, and fracture location were varied in a series of scenarios with one question: should this patient be offered SSRF? A heat map of response was then generated and the scenario which best approximately clinical equipoise (50% of respondents would offer SSRF, 50% would not) was identified (Figure 1). In this case, it was a patient aged 18–75, with no or mild TBI, and ≥2 pulmonary derangements. This hypothetical patient served as the template from which inclusion criteria for our multicenter RCT were selected. Importantly, it was decided a priori that this trial would not include patients with flail chest, because it was believed that grouping patients with and without this diagnosis would complication the interpretation of its findings.
Determining feasibility
Because multicenter RCTs are extraordinarily timely and expensive, it is imperative to estimate the likelihood that any trial could be realistically completed prior to proceeding. The first step in this process involves calculation of a sample size, which, in turn, requires selection of a primary outcome variable. In general, the total sample size should be based upon the outcome which will require the largest number of subjects; this outcome is typically a binary, categorical parameters (e.g., pneumonia) as opposed to a continuous parameter (e.g., numeric pain score). For a RCT, sample sizes must be adjusted to reflect both declination to participate and attrition. Although the literature on this topic is sparse, data suggest that the declination rate for RCTs in general is approximately 25–50% (21,22), but may reach as high as 75% in studies that randomize patients to surgery vs. no surgery (23,24). Furthermore, attrition from multicenter surgical trials has been reported to be anywhere from 10–33% (25). Thus, in order to be feasible, a RCT that requires 100 subjects to be adequately powered to answer the research question should identify centers that, in sum, treat at least 200 patients over the study period who otherwise meet inclusion criteria. Stated differently, the minimum necessary sample size to achieve the desired statistical power should be doubled to account for both declinations to participate and attrition. If the involved centers do not treat this number of patients over the study period, the study will likely not be feasible.
In general, conducting a multicenter RCT through a parent organization facilitates identification of centers, dissemination of information, and institutional review board (IRB)/contracting issues. Because a standardized protocol for both operative and non-operative management must be employed for any RCT, an assessment of potential centers’ likelihood of complying with said protocols should be made. In the case of CWIS NON-FLAIL, a standardized survey was sent to interested investigators assessing their center’s volume of rib fracture patients who would meet eligibility, as well as practice patterns regarding both operative and non-operative management of patients (https://www.surveymonkey.com/r/cwisRCT). These data were used to identify centers that would best match the intentions of the RCT.
Particularly important to identifying satellite centers is a thoughtful inquiry into whether or not the surgeon researchers could in good faith randomize patients to either operative or non-operative management. Several centers were ultimately turned down from CWIS NON-FLAIL because they believed strongly (despite a relative absence of data!) that surgery was highly effective in the target population. Beyond the obvious ethical conflict that would be experienced by the investigators, it is highly likely that involvement of such centers would introduce bias towards favorable outcomes in patients randomized to surgery.
Standardizing the research protocol
Beyond the efficacy of SSRF, many more basic uncertainties remain in the field of chest wall injury. For example, there remains a lack of standardized nomenclature to describe the anatomy of rib fractures with respect to location, degree of displacement, and definition of flail chest. Furthermore, although the technique of SSRF has evolved greatly over the last 10 years (16), there is no one agreed upon operative protocol, and approaches range from muscle sparing thoracotomy (26) to completely thoracoscopic repair (27). Finally, there is currently no validated tool to measure rib-fracture specific quality of life.
In order to overcome these limitations, it is recommended that a protocol, wherever possible, use objective, validated measurements of both predictor and outcome variables. Both the Blunt Pulmonary Contusion Score (28) and the RibScore (29) represent examples of such tools. Calculators to assist in determining scores should be relatively accessible, either through embedment into the data collection tool or on a trial website, so as to minimize user error. In cases where there is no universally agreed upon definition, the study personnel should choose (or make up) one, and use it exclusively. Moving forward, establishment of standard tools for defining rib fractures, complications of surgery, and quality of life, will achieve a baseline by which future studies can be compared to each other.
Funding
It is impossible to conduct a multicenter RCT of SSRF without funding. Regulatory costs, research personnel, consent translations into language other than English, and additional tests beyond what may be standard of care in certain institutions (e.g., pulmonary function tests) represent some examples of costs that should be anticipated. The majority of study costs will likely be related to the IRB (30). Although IRB fees for the initial application are typically anticipated, additional costs such as those associated with protocol amendments, adverse event reporting, and routine renewal (usually yearly), are often overlooked and should be considered and quantified. Furthermore, one major limitation to the IRB process is the lack of centralization; accordingly, each satellite center will require independent review, including charges. Total IRB costs per institutions for non-federally-sponsored trials may approach the tens of thousands per center (30).
The issues of payment responsibility for healthcare services typically arises in RCTs that randomize patients to an intervention, and it is best to anticipate and investigate this issue before enrollment begins. Specifically, both hospitals and IRBs may request that the research study pays for the costs of surgery in patients randomized to SSRF. In fact, this request was made by several satellite site IRBs in the course of CWIS NON-FLAIL. However, in all but one case, the lead study center was able to successfully negotiate that the research study should not pay for the cost of the operation. In the remaining case, unfortunately, the study center was ultimately unable to participate in the randomization arm of the study, despite the appropriate infra-structure and a motivated research team.
There is no universal, legal precedent to dictate payment responsibility for surgical procedures performed in the course of a research trial, and the investigators of CWIS NON-FLAIL believed strongly that patients should be responsible for paying the costs associated with their treatment arm, be it either operative or non-operative. The first reason for this position is that there is no clear evidence that surgery is more expensive than non-operative management of severe rib fractures. In fact, there is some data to suggest that operative management is actually less expensive, as compared to non-operative management, from both the patient and the hospital’s perspective (19,31,32). Why, then, would a patient be asked to pay for the costs of one (potentially less expensive) treatment arm, but not the other? It is interesting that the requests for study payment made by the IRBs were always specific to the costs of the operation, and did not include the costs of additional therapies, such as loco-regional anesthesia, hospitalization charges, or payment for complications that may arise in either arm of the trial. If there is true equipoise between treatment arms, then the research trial should not have to selectively pay for costs incurred in any one group.
A second important consideration with respect to payment responsibility, is that of coercion. It stands to reason that patients in dire financial circumstances (e.g., hospitalized after a major trauma without insurance) may desire a surgical intervention but fear the financial repercussions. Such patients may then elect to potentially receive surgery only because it is free. By contrast, if the patients incur the cost of their care, either operative or non-operative, they could more objectively decide whether or not to participate.
In general, federal research funding, including that from the National Institutes of Health, has decreased dramatically over the last 10 years (33). Both regional and national surgical societies offer research fellowships, but these are typically at an amount far less than in required to execute a multicenter RCT. Finally, investigator-initiated studies funded via industry represents a viable option for conducting a multicenter RCT. However, this funding source requires careful identification and management of any potential financial conflicts of interest (COI, see below section on COI). A general estimation for a surgical trial of this magnitude would be $5,000–$10,000 per subject.
A clear, itemized budget is recommended at the time of the funding application. Most institutions incorporate indirect costs into the budget, and may also add additional administrative costs. For these reasons, involvement of a grant and contracting office, if it exists, is prudent prior to submitting any request for funding, as it is more cumbersome to retroactively manipulate the estimated costs. Finally, a clear expectation of both amount and schedule of payments to the satellite sites will minimize the occurrence of future disputes.
IRBs, grants and contracting, and legal offices
IRBs have been instrumental in protecting the rights and welfare of human research subjects. Formal review procedures for human subjects were originally developed in direct response to research abuse over the course of the 20th century. Notorious examples of this abuse include the Nazi physician experiments, Tuskegee Syphilis Study, and human radiation experiments. Common to these studies is the targeting of vulnerable populations, including prisoners, children (or fetuses), and the mentally disabled.
In response to these atrocities, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research drafted the Belmont Report, issued on September 30th, 1978, which detailed the three basic ethical principles for using human subjects in research: respect for persons, beneficence, and justice. In 1974, the National Research Act formally mandated independent review of all studies involving human subjects. In the United States, the Food and Drug Administration and Department of Health and Human Services Office for Human Research Protections, guided by this document, have empowered IRBs to approve, require modifications, or disapprove research. Central to IRB review are the notions of minimization of risk, informed consent, equitable selection of participants, protection of privacy, and data monitoring. Between 2005 and 2009, the number of IRBs has increased from 491 to almost 4,000 (34).
Although IRBs serve to recognize and mitigate unnecessary risk to participants, their existence introduces several logistical hurdles to conducting clinical trials. Furthermore, many researchers contend that, although the original intention of this process was sound, the contemporary IRB review process imposes certain costs that do not add to the protections afforded to research participants and may threaten the viability of research (35). The first cost is time. A recent study of time to IRB approval at 10 Veterans Affairs Medical Centers reported an average review time to be nearly four months (36); review times substantially longer than this have also been reported (37). Accordingly, the IRB process should be initiated as early as possible in the course of a trial. In many cases, the IRB review process cannot start prior to paying the IRB fee which, in turn, may be conditional upon procurement of funding. However, most IRBs provide generic application forms that can be completed so that they are ready to be submitted as soon as funding is secured. Finally, a minimum of three months should be added to the trial timeline to account for IRB review and approval.
The second concern, which is of particular relevance to a multicenter trial, is regional variability in IRB conduct. A major limitation of the current IRB review process remains the lack of centralization. As such, different IRBs frequently disagree on both the decision to accept or reject a protocol (38) and (sometimes more frustratingly) relatively minor protocol details (39). In the case of any protocol modifications at the lead study site, the revised protocol must be approved at each satellite site, beginning anew the sometimes lengthy review process. In the case of the NON-FLAIL trial, IRB of two institutions within the same city resulted in different determinations (accept with modifications and reject) and also requested that different changes were made to the protocol. In a second example of regional IRB variability, one IRB determined that a standardized patient consent video was a model example of informed consent, whereas another deemed it “coercive” and disallowed it from use at that center.
From the investigator’s perspective, there are several steps to mitigate this issue of variability. The first is to secure IRB approval at the lead study center first. The IRB approved protocol can then be disseminated to the satellite sites. If not, any changes to the protocol requested by the lead IRB will require that each satellite center revisit IRB approval. The second is to make the study protocol as generic as possible while still preserving the integrity of the protocol. One example of this balance is that the CWIS NON-FLAIL protocol initially required that routine bronchoscopy be performed during SSRF. Because many centers did not routinely perform bronchoscopy, their IRBs determined that this was not standard of care, which then began issues of both consent and payment. Ultimately, the lead investigator decided that whether or not an intra-operative bronchoscopy was performed was not important enough to the study to delay it several months for the satellite center IRBs to come into alignment. The protocol language was revised to make the procedure optional at centers where it was considered standard of care.
Additionally, even though it is ultimately the participating center’s responsibility to submit their own application and uphold review board communications, a lead site is still an integral player is each subsite’s submission. It is necessary for the lead site to assist in the process to ensure standardization across all submissions. This process includes review and approval of all materials being submitted (e.g., application, informed consent forms, and standard of procedure forms) and assistance in answering subsequent IRB request until approval.
The next layer of complexity lies with legal. Each participating center must also be legally permitted to do business with and send data to the lead site. If there is grant funding awarded for the study and PHI to be shared, the business component becomes more precarious as contracts not only need to exist between all sites but also the sponsor (40). This leads to time consuming negotiations between legal entities to draft, redline, and execute a clinical trial agreement (CTA). Additionally, the CTA, or other equivalent contract, is often required to be finalization even before the site is given permission to submit their study for IRB review. This is why forewarning is due to understand one’s institutional requirements and to allow for extra time for these processes to transpire. Special attention needs to be allotted to draft an appropriate contract. One example from CWIS NON-FLAIL involved a CTA that was drafted and signed too hastily was an instance that resulted in a severe delay and near exclusion of an eager site from study participation. The misstep was in reference to the type of data repository employed by the study. Once the discrepancy was identified, the satellite site had to recommence contract negotiations; a process that consumed approximately 7 months.
Data sharing contracts are also required when interacting data with members of a Data Safety Monitoring Board (DSMB), and some institutions require contracts with the data repository itself, especially when PHI is involved. This exemplifies that stringent regulation exist when it comes to protecting patient data and institutional risk and highlights the attention that is needed for the proper amount of time to allow for these contracts to develop.
Given the complexity of the regulatory negotiations involved in conducting a multicenter trial, it is imperative that personal, positive connections are formed between members of the research team, IRB, and legal departments. Face to face communications, including satellite site visits, whenever possible, are generally more powerful then electronic mail correspondences. Compiling a standardized “bundle” of documents to assist satellite centers with the aforementioned regulatory tasks will also expedite the process. Finally, although investigators may believe that they have the moral high ground in negotiations with regulatory agencies, resistance (either overt or passive aggression) almost always serves only to negatively impact the study. Our experience over the course of CWIS NON-FLAIL was one of both patience and humility.
Identifying and managing financial conflict of interest
Trials of SSRF by definition will involve collaboration with industry. Furthermore, it is highly likely that one or more study investigators will have a financial relationship with industry outside of the research trial. Examples of such interests include teaching at industry sponsored courses, consulting with industry to develop new products, and prior or current research funding from industry. Any of these scenarios may potentially cause a conflict, whether conscious or not, between personal financial and research interests.
In general, clinicians with potential conflicts of interest tend to not disclose them adequately; this concern is particularly apparent among physicians receiving the highest payment amounts from industry (5). This concerning finding underscores the importance of full disclosure of any potential conflicts of interest by study personnel. Regardless of the researcher’s own belief in their personal integrity, patients deserve to know who is funding the trial, and if the researchers are receiving separate payments from industry directly involved in the surgical arm of the trial.
Several safeguards are recommended to guard against potential conflicts of interest. First, almost every major medical center has an office of compliance, in addition to the IRB, that requires completion of annual disclosure forms and, in the case of a potential conflict, meeting with the researcher to formulate and enact a management plan. In general, a potential conflict of interest becomes an issue when the individual has received over $5,000 in compensation from industry that is relevant to the trial.
The first step in managing potential conflict of interest is realizing that it is acceptable to have independent relationships with industry, provided that an appropriate management plan is in place that is mutually agreeable to the researcher and the institution. The three overarching principles of the management plan are disclosure, independent review, and generic choice of fixation hardware. Disclosure of both funding source and financial relationships of the researchers to potential subjects is standard. In the case of CWIS NON-FLAIL, the sentence, “Dr. XX serves as a paid educator for XX, one of the manufactures of a rib plating system that may be used in your surgery.” The second principle is independent review. This review could take the form of a data safety monitoring board, independent biostatistician, or parent society through which the protocol is vetted. In the case of CWIS NON-FLAIL, data analysis will be repeated by an independent biostatistician. Finally, the investigators felt strongly that, although research funding for CWIS NON-FLAIL was provided by one company, any plating system could be used by the satellite sites to perform SSRF. In this way, there was no direct financial incentive to the study sponsor or researchers for patients to be enrolled.
Many investigators (understandably) react negatively to inquiries by their institution’s regulatory office. Such inquiries may be viewed as questioning the researcher’s personal integrity, or ability to objectively conduct research in the best interest of the patients. However, it is important to remember that the purpose of such offices is to protect patients against nefarious research that, although may not be taking place at your institution, certainly has in the past and continues to happen today. Approaching negotiations with the IRB, legal, and regulatory offices in a positive and deferential manner will ultimately maximize the likelihood of a positive, timely outcome.
Multicenter onboarding and study conduct
Once IRB approvals are implemented and institutional legalities are executed, the lead site may begin training the satellite site on study conduct. The focus again is standardization across all elements including patient screening, enrollment, data collection, and addressing of any unanticipated problems (UAPs). A site visit or site initiation call is required to reinforce this appropriate etiquette to avoid serious issues like wrongful enrollment, patient harm, or neglect in reporting of adverse events. Offenses in any of these areas can lead to premature study termination (41). In our experience, the best way to assure this level of safety was to allocate a single point of contact (POC) to walk-through the first five enrollments with the research team at each site and to troubleshoot any data collection issues, as they occurred. The POC also is the primary resource when it comes to providing guidance for any UAPs.
The concept of UAPs are a well-established entity recognized by every IRB and contrary to their name, are anticipated to occur; addressing questions as they arise, can help avoid more serious adverse events from developing. Even though one can strive to combat UAPs from happening, inevitably there will be occurrences. These are well received by the IRB if reported appropriately (42). Adverse events are problems of a more serious nature and may require reporting to the IRB within 5 days, thus it is imperative for the lead site to remain in constant communication with each subsite to become aware of these incidents. While it is the individual site’s responsibility to uphold correspondence with their local IRB, the lead institution must provide oversight to ensure that the study is not in violation of continuation. None of these safety assurances occur in isolation so the collective presentation of a site initiation, training, and continuous subsite communication all lay within the collaborative effort of each participating center.
Conclusions
Multicenter RCTs represent the gold standard of research into the efficacy of SSRF, and several of them are still needed to address different patient populations and operative techniques. However, although many academic surgeons frequently (and somewhat haphazardly) quip the line “we need a RCT to answer that,” the process of designing and executing such a trial constitutes a herculean effort. Most RCTs fail because of poor planning. Specifically, they are underpowered, underfunded, understaffed, and run out of time. Understanding up front that a multicenter RCT of SSRF will involve a multi-year, multi hundred thousand dollar commitment will help to mitigate these risks. Organization of future trials through parent organizations (e.g., CWIS) will aid greatly in both standardization and execution. Finally, designating one or more research coordinators whose full-time job is to maintain the interest in and integrity of the trial is necessary to achieve a positive outcome. The acquisition of evidence surrounding the efficacy of SSRF will depend on the dedication and perseverance of surgical researchers to overcome the myriad obstacles mentioned herein and arrive at the truth.
Acknowledgements
We would like to thank the members and staff of the Chest Wall Injury Society and Denver Health for their efforts in making this mutli-center RCT possible. We would also like to thank all the participating centers of the trial, and those at the Colorado Multiple Institutional Review Board (COMIRB), for finding a way to work with us, instead of against us.
Footnote
Conflicts of Interest: Dr. FM Pieracci is the 2018 president of the Chest Wall Injury Society and a paid educator by DePuy Synthes, for which he is funded for teaching other surgeons the technique of rib plating at national courses. The other authors have no conflicts of interest to declare.
References
- Fitzpatrick DC, Denard PJ, Phelan D, et al. Operative stabilization of flail chest injuries: review of literature and fixation options. Eur J Trauma Emerg Surg 2010;36:427-33. [Crossref] [PubMed]
- Kane ED, Jeremitsky E, Pieracci FM, et al. Quantifying and exploring the recent national increase in surgical stabilization of rib fractures. J Trauma Acute Care Surg 2017;83:1047-52. [Crossref] [PubMed]
- Kasotakis G, Hasenboehler EA, Streib EW, et al. Operative fixation of rib fractures after blunt trauma: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2017;82:618-26. [Crossref] [PubMed]
- Burlew CC, Davis KA, Fildes JJ, et al. Acute care surgery fellowship graduates' practice patterns: The additional training is an asset. J Trauma Acute Care Surg 2017;82:208-10. [Crossref] [PubMed]
- Ziai K, Pigazzi A, Smith BR, et al. Association of Compensation From the Surgical and Medical Device Industry to Physicians and Self-declared Conflict of Interest. JAMA Surg 2018;153:997-1002. [Crossref] [PubMed]
- Mayberry JC, Ham LB, Schipper PH, et al. Surveyed opinion of American trauma, orthopedic, and thoracic surgeons on rib and sternal fracture repair. J Trauma 2009;66:875-9. [Crossref] [PubMed]
- Smith GC, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ 2003;327:1459-61. [Crossref] [PubMed]
- Pieracci FM, Agarwal S, Doben A, et al. Indications for surgical stabilization of rib fractures in patients without flail chest: surveyed opinions of members of the Chest Wall Injury Society. Int Orthop 2018;42:401-8. [Crossref] [PubMed]
- Pieracci FM, Majercik S, Ali-Osman F, et al. Consensus statement: Surgical stabilization of rib fractures rib fracture colloquium clinical practice guidelines. Injury 2017;48:307-21. [Crossref] [PubMed]
- , accessed November 3rd, 2016.www.gradeworkinggroup.org
- Pieracci FM, Coleman J, Ali-Osman F, et al. A multicenter evaluation of the optimal timing of surgical stabilization of rib fractures. J Trauma Acute Care Surg 2018;84:1-10. [Crossref] [PubMed]
- Pieracci FM, Lin Y, Rodil M, et al. A prospective, controlled clinical evaluation of surgical stabilization of severe rib fractures. J Trauma Acute Care Surg 2016;80:187-94. [Crossref] [PubMed]
- Tanaka H, Yukioka T, Yamaguti Y, et al. Surgical stabilization of internal pneumatic stabilization? A prospective randomized study of management of severe flail chest patients. J Trauma 2002;52:727-32; discussion 32. [Crossref] [PubMed]
- Granetzny A, Abd El-Aal M, Emam E, et al. Surgical versus conservative treatment of flail chest. Evaluation of the pulmonary status. Interact Cardiovasc Thorac Surg 2005;4:583-7. [Crossref] [PubMed]
- Marasco SF, Davies AR, Cooper J, et al. Prospective randomized controlled trial of operative rib fixation in traumatic flail chest. J Am Coll Surg 2013;216:924-32. [Crossref] [PubMed]
- Pieracci FM, Rodil M, Stovall RT, et al. Surgical stabilization of severe rib fractures. J Trauma Acute Care Surg 2015;78:883-7. [Crossref] [PubMed]
- Leinicke JA, Elmore L, Freeman BD, et al. Operative management of rib fractures in the setting of flail chest: a systematic review and meta-analysis. Ann Surg 2013;258:914-21. [Crossref] [PubMed]
- Coughlin TA, Ng JW, Rollins KE, et al. Management of rib fractures in traumatic flail chest: a meta-analysis of randomised controlled trials. Bone Joint J 2016;98-B:1119-25. [Crossref] [PubMed]
- Swart E, Laratta J, Slobogean G, et al. Operative Treatment of Rib Fractures in Flail Chest Injuries: A Meta-analysis and Cost-Effectiveness Analysis. J Orthop Trauma 2017;31:64-70. [Crossref] [PubMed]
- Slobogean GP, MacPherson CA, Sun T, et al. Surgical fixation vs nonoperative management of flail chest: a meta-analysis. J Am Coll Surg 2013;216:302-11.e1. [Crossref] [PubMed]
- Jonsdottir H, Ingadottir TS. Reluctance of patients with chronic obstructive pulmonary disease in its early stages and their families to participate in a partnership-based self-management trial: A search for explanation. Chron Respir Dis 2018;15:315-22. [Crossref] [PubMed]
- Halpern SD, Karlawish JH, Casarett D, et al. Hypertensive patients' willingness to participate in placebo-controlled trials: implications for recruitment efficiency. Am Heart J 2003;146:985-92. [Crossref] [PubMed]
- Creel AH, Losina E, Mandl LA, et al. An assessment of willingness to participate in a randomized trial of arthroscopic knee surgery in patients with osteoarthritis. Contemp Clin Trials 2005;26:169-78. [Crossref] [PubMed]
- Ohmann C, Deimling A. Attitude towards clinical trials: results of a survey of persons interested in research. Inflamm Res 2004;53 Suppl 2:S142-7. [Crossref] [PubMed]
- Fabricant L, Ham B, Mullins R, et al. Prospective clinical trial of surgical intervention for painful rib fracture nonunion. Am Surg 2014;80:580-6. [PubMed]
- Ali-Osman F, Mangram A, Sucher J, et al. Geriatric (G60) trauma patients with severe rib fractures: Is muscle sparing minimally invasive thoracotomy rib fixation safe and does it improve post-operative pulmonary function? Am J Surg 2018;216:46-51. [Crossref] [PubMed]
- Pieracci FM, Johnson JL, Stovall RT, et al. Completely thoracoscopic, intra-pleural reduction and fixation of severe rib fractures. Trauma Case Rep 2015;1:39-43. [Crossref] [PubMed]
- Tyburski JG, Collinge JD, Wilson RF, et al. Pulmonary contusions: quantifying the lesions on chest X-ray films and the factors affecting prognosis. J Trauma 1999;46:833-8. [Crossref] [PubMed]
- Chapman BC, Herbert B, Rodil M, et al. RibScore: A novel radiographic score based on fracture pattern that predicts pneumonia, respiratory failure, and tracheostomy. J Trauma Acute Care Surg 2016;80:95-101. [Crossref] [PubMed]
- Silberman G, Kahn KL. Burdens on research imposed by institutional review boards: the state of the evidence and its implications for regulatory reform. Milbank Q 2011;89:599-627. [Crossref] [PubMed]
- Majercik S, Wilson E, Gardner S, et al. In-hospital outcomes and costs of surgical stabilization versus nonoperative management of severe rib fractures. J Trauma Acute Care Surg 2015;79:533-8; discussion 8-9. [Crossref] [PubMed]
- Bhatnagar A, Mayberry J, Nirula R. Rib fracture fixation for flail chest: what is the benefit? J Am Coll Surg 2012;215:201-5. [Crossref] [PubMed]
- Hu Y, Edwards BL, Brooks KD, et al. Recent trends in National Institutes of Health funding for surgery: 2003 to 2013. Am J Surg 2015;209:1083-9. [Crossref] [PubMed]
- Catania JA, Lo B, Wolf LE, et al. Survey of u.s. Human research protection organizations: workload and membership. J Empir Res Hum Res Ethics 2008;3:57-69. [Crossref] [PubMed]
- Emanuel EJ, Wood A, Fleischman A, et al. Oversight of human participants research: identifying problems to evaluate reform proposals. Ann Intern Med 2004;141:282-91. [Crossref] [PubMed]
- Varley PR, Feske U, Gao S, et al. Time required to review research protocols at 10 Veterans Affairs Institutional Review Boards. J Surg Res 2016;204:481-9. [Crossref] [PubMed]
- Burman W, Breese P, Weis S, et al. The effects of local review on informed consent documents from a multicenter clinical trials consortium. Control Clin Trials 2003;24:245-55. [Crossref] [PubMed]
- Vick CC, Finan KR, Kiefe C, et al. Variation in Institutional Review processes for a multisite observational study. Am J Surg 2005;190:805-9. [Crossref] [PubMed]
- Green LA, Lowery JC, Kowalski CP, et al. Impact of institutional review board practice variation on observational health services research. Health Serv Res 2006;41:214-30. [Crossref] [PubMed]
- Mello MM, Clarridge BR, Studdert DM. Academic medical centers' standards for clinical-trial agreements with industry. N Engl J Med 2005;352:2202-10. [Crossref] [PubMed]
- Champion HR, Fingerhut A, Escobar MA, et al. The role of data and safety monitoring in acute trauma resuscitation research. J Am Coll Surg 2007;204:73-83. [Crossref] [PubMed]
- Byerly WG. Working with the institutional review board. Am J Health Syst Pharm 2009;66:176-84. [Crossref] [PubMed]


