Effect of pharmaceutical care on medication adherence and hospital admission in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled study
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease and a main cause of morbidity and mortality worldwide (1). In China increasingly high prevalence of COPD will impose greater and greater burden on whole society in years to come as Chinese is aging and pollution is deteriorating (2,3). Although COPD can be treated by medications, poor drug adherence can dramatically counteract the therapeutic efficacy of medications. It is common in patients with COPD that they fail to keep an appointment with their physician, fail to take drugs as prescribed, or even do not take them at all. Because COPD is not reversible, the purpose of pharmacological treatment is to reduce exacerbations and symptoms. A recent systematic review revealed that more than 40% of the COPD patients were not compliant to prescribed drugs (4), leading to a high rate of exacerbation and poor health related quality of life (HRQoL) (5,6).
Despite its importance, medication adherence is an individual patient behavior that is difficult to improve (7). Previous clinical randomized controlled trials examining the effects of on adherence in patients with COPD have shown conflicting results. Khdour MR et al. (8) and Jarab AS et al. (9) reported clinical pharmacists with an individualized education program and telephone follow-up continued one year can improve adherence and reduce hospitalization. Garcia-Aymerich J et al. (10) reported nurses with an individualized care plan and telephone follow-up at discharge for one year can also improve adherence. However, there was no significance on adherence between the control and intervention group in two studies (11,12). Gallefoss and colleagues (11) developed face-to-face education program delivered by a nurse and physiotherapist, no significant difference in medication adherence was found compared usual general practitioner care at 12-month follow-up. Solomon and colleagues (12) used a face-to-face and telephone pharmaceutical care completed by a pharmacist and pharmacy residents, but there was no improvement on adherence between intervention and control groups at 6-month follow-up.
A systematic review found that educational interventions and memory aids were two major types of strategies in older patients to optimize medication adherence (13). Ross JS. held that successful interventions on adherence are often labor intensive and comprehensive, and direct advice to COPD patients from pharmacists may be particularly promising because of their specialized training and knowledge of medications and availability to the patients (7). In this study, we developed our pharmaceutical care program and investigated whether and how our interventions improve the current poor adherence in COPD patients.
Methods
Patients
Participants were enrolled from The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, from February 7 to August 10, 2012. The entrance criteria were as follow: (I) stable COPD (respiratory symptoms and medication unchanged for at least 4 weeks before enrollment); (II) a post-bronchodilator forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) ratio of less than 0.70 and an FEV1 between 25% and 79% of predicted value; (III) at least two consecutive visits to our hospital for the treatment of COPD; (IV) no participation in a respiratory rehabilitation in the past year; and (V) no previous diagnosis of asthma, dementia, uncontrolled psychiatric disease and severe heart, liver, and kidney disease. The exclusion criteria were: (I) adherence patients (taken above 80% of the daily dose prescribed); (II) refusal to participate the study. All COPD patients having fulfilled the entrance criteria, and having no exclusion criteria present, were invited to participate. The study was approved by local ethics committees and was done in compliance with the Declaration of Helsinki.
Study design
This was a prospective randomized controlled study completed by January 31, 2014. The pharmacists were blinded to the randomization codes, which were computer generated and sealed in envelopes labeled with consecutive numbers. The envelopes were opened and patients were allocated to the control or pharmaceutical care group.
A comprehensive pharmaceutical care program had been developed by our clinical pharmacists based on relative researches (14,15). It was chiefly composed of provision of individualized education and a series of telephone counseling. Patients were individualized educated (20-30 minutes per session, 5-6 sessions) in a structured fashion step by step during the clinic visit. The interview contents included effective use of respiratory devices, pathophysiology of the disease, interpretation of medical testing and rationale for medication. After each interview, medication management records for patients were established that evaluated each participant’s preferences and analyzed possible barriers to medication adherence. The frequency of a 10-minute telephone call was based on the results of last interview (maximum reach 12 sessions, weekly call at first month in 25% patients of pharmaceutical care group), but generally telephone call (4-5 sessions) at the midpoint between two clinic visits. During telephone counseling, the pharmacist asked about the patient’s treatment effects, clarified any misconceptions, explained the nature of any side effects and reminded patients of their next clinical appointment. The pharmaceutical care program continued for half a year. Patients in the control group received general counseling, but no individualized education and follow-up telephone counseling.
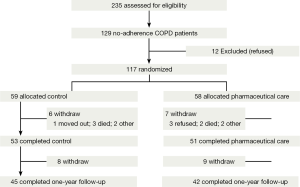
An interview was held by clinical pharmacist to investigate hospital admissions and evaluate severe exacerbations at one-year follow-up (Figure 1).
Outcome measures
The primary endpoint was medication adherence which was measured by pill counts plus direct interview. Our main measurement method of medication adherence is pill counts. In order to increasing its accuracy, structured questionnaire was developed based on Wu et al.’s study (14). The pharmacist asked the patient to describe their regimen prescribed at their last visit by drug dosages (number of pills) and frequency when patients were given drug samples. They were asked whether they had missed any medication, or changed their regimens in terms of dose and frequency. This information was checked against the dispensing information and gave the pharmacist a reasonable impression of the patient’s degree of adherence. We defined those as adherence patients if they had taken beyond 80% of the daily dose prescribed, and those as non-adherence patients if they failed to do so. Patient adherence was scored by the total number of prescribed drugs a patient took for a month and expressed as a percentage. A patient who complied with all prescribed drug had an adherence score of 100%, whereas one who complied with only three of the six drugs had an adherence score of 50%. Medication adherence was assessed by clinical pharmacists who have been specifically trained for the study before intervention, at 1- and 6-month pharmaceutical care, and at one-year follow-up.
Secondary endpoints included the severe exacerbation rate and HRQoL. A severe exacerbation of COPD was defined as a hospitalization due to an acute COPD attack (16). Hospital admission was defined as (I) hospital stay of any duration in an acute care bed; (II) or day hospital stay of over 2 consecutive days (17). HRQoL was assessed by disease-specific St George’s Respiratory Questionnaire (SGRQ, using a validated Chinese version) (18). SGRQ consists of three domains: (I) respiratory symptoms; (II) activities (a measure of the activities that cause or are limited by breathlessness); and (III) impacts (a measure of the overall disturbance of daily life, social function, and well-being). The scores range from 0 to 100, with a lower score indicating a better quality of life. SGRQ was measured by clinical pharmacists before intervention and at 6-month pharmaceutical care, respectively.
Statistical analysis
Accurate calculation of sample size had been an issue of great attention when planning the trial, but we were unable to do so because of a scarcity of reference data from previously published studies. Consequently, the sample size was determined on the basis of experiences of Chinese clinical pharmacists. The sample size was estimated to be powered for this study.
All statistical analyses for baseline characteristics and outcomes were done on an intention-to-treat base. We used SPSS10.0 for all analyses. Data are presented as means ± SD or as percentages within groups. The student’s sample t-test and χ2 test were used to compare groups. Exacerbation rate was also analysed with a regression model including interactions between intervention and covariates (such as history of exacerbation, FEV1, the number of taken inhaled corticosteroids and long acting bronchodilators, and quality of life). A two sided value of P<0.05 was considered to be significant.
Results
We interviewed 235 eligible patients with COPD from the outpatient clinic, 106 (45%) of them were adherence patients. One hundred and twenty-nine non-adherence COPD patients were recruited for inclusion in the study, 12 patients declined to participate. Their main reason for refusal was that they felt the evaluation a serious inconvenience. One hundred and seventeen patients are randomized evenly to a control group (59 patients) and a pharmaceutical care group (58 patients) (Figure 1).
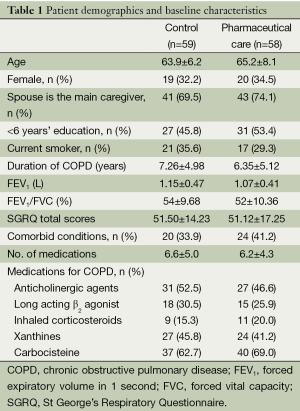
The two groups were similar in socio-demographic, clinical, functional variables, and respiratory medication (P>0.10, Table 1). The number of patients taking medications that decrease exacerbation (inhaler corticosteroids, anti-cholinergic agents, and long-acting β2 agonist) was similar between the pharmaceutical care group and control group (41 vs. 37, P=0.21). During 6-month intervention, the patients in the pharmaceutical care group received 5.48±3.62 sessions individualized education and 5.76±4.12 sessions telephone counseling.
Full table
At 6-month intervention and 1-year follow-up, the pharmaceutical care group exhibited significant higher adherence score (%) than the control group, but there was no such a significant difference between the two groups at 1-month (Table 2). In the pharmaceutical care group, the adherence score at 1-year follow-up was significantly decreased compared with that at 6-month intervention (73.4±11.1 vs. 66.5±8.6, P=0.042).

Full table
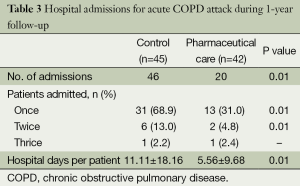
Patients admissions due to acute exacerbations of COPD in the year preceding study entry were similar between the both groups (41 vs. 38, P=0.45). At 1-year follow-up, 46 severe acute exacerbation in the control group resulted in a hospital admission while 20 ones in the pharmaceutical care group, yielding a 56.5% reduction in hospital admissions (P=0.01). Significantly more patients in the control group had one hospital admission and two hospital admissions during the 1-year follow-up respectively (Table 3). By analysing the covariance factors in the regression model, no factors were found to significantly affect COPD exacerbation.
Full table
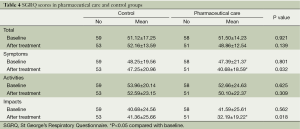
Baseline HRQoL scores on the SGRQ were comparable between the control and pharmaceutical care groups on each of the subscales and the total scores (Table 4). At 6-month intervention, scores on symptoms and impacts subscales in the pharmaceutical care group were significantly improved compared with those in the control group respectively, while activities subscale and total scores were not significant difference between the two groups (Table 4).
Full table
Discussion
Our study showed that individualized 6-month pharmaceutical care improved the medication adherence in patients with COPD. We found that the medication adherence of the pharmaceutical care group was superior to that of the control group at 1-year follow-up, suggesting the therapeutic effects might be sustainable.
Some studies used questionnaires to measure medications adherence for patients with COPD (8-10). Khdour MR et al. (8) and Jarab AS et al. (9) administered Morisky Scale used widely in many kind of diseases, while Garcia-Aymerich J et al. (10) used Inhaler Adherence Scale suited for inhaler drugs. Morisky Scale measures adherence through four Yes/No response items, reflects the number of ways medication omission can occur: forgetting, carelessness, stopping when feeling better and stopping when feeling worse (19). Although these scales are relative easy to measure, questioning the patients can be biased by inaccurate patient recall or social desirability resulting in the health care provider’s overestimating the patient’s adherence (20). Pill counts measurement is a relative objective and one of the most commonly used methods for evaluating adherence, but this method may provide wrong information (i.e., switch medicines between bottles before visits in order to be following the regime), and provides no information on other aspects of taking drug (i.e., dose timing) (21). In our study pill counts plus direct interview maximized the accuracy of measuring medication adherence (22).
There are many barriers to medication adherence for patients with COPD. In response to a questionnaire, typical reasons cited by patients for not taking their drugs included high costs for drugs and taking medication based on their feeling (e.g., “it does not do any good”) (23). This intentional non-adherence is often due to the patient’s misunderstanding the clinician’s instructions. Although proper education ensure the patient is fully informed about the important aspects of their treatment regimen, generally educational interventions, such as mailed patient educational material and brief interaction, do not consistently improve medication adherence (13). No education effects on medication adherence by Gallefoss and colleagues (11) may be due to the short term intervention which consisted of two 2-hour group sessions and 1 to 2 individual sessions. Our study showed that medication adherence improved significantly at 6-month pharmaceutical care, but not at 1-month pharmaceutical care, suggesting that long-time intervention might be sufficient to alleviate patients’ concern. Besides the patients’ understanding, their motivation and expectation about the success likelihood of medical intervention are critical factors to medications adherence (24). As patients were intervened by outpatient pharmacists mainly in community or ambulatory care settings, no significant changes in adherence scores by Solomon and colleagues (12) may be due to lacking of motivation and expectation of patients. In our face-to-face pharmaceutical care intervention, one of the goals is help the patients understand and believe the treatment is good and fit for him.
After the patients understand and are convinced of the treatment of COPD, some of them still exhibit poor medication adherence. This difficulty may be attributable to a busy schedule, lack of attention to detail, and inadvertently forgetting to take their medications. Periodic reinforcements are necessary to improve medication adherence. Marteau TM et al. (25) held that much automatic behavior is cued by environmental stimuli, resulting in actions that are largely unaccompanied by conscious reflection. In our study, medication adherence in the pharmaceutical care group at 1-year follow-up decreased compared with that at 6-month pharmaceutical care, suggesting that continuous support from clinical pharmacist is indispensable to changing negative health behaviors of patients.
An important finding in the present study was the 56.5% reduction in hospitalization over the control group at 1-year follow-up. Since exacerbation of COPD can worsen health status and fasten disease progression, reduction of exacerbation rate is a key target for intervention (16). Some researchers reported a reduction in hospital admission as a result of educational interventions (8-10,12,17). In education plan of these studies, researchers emphasized prompt initiation of antibiotic and oral corticosteroid medication for COPD exacerbations besides improving medication adherence. Rees PJ argued the self-management program might be the major reason for the reduction in hospital admissions with exacerbations (26). In our study, reduction in hospital admissions might have been more attributable to reduction in severe exacerbation.
In addition to preventing COPD exacerbations, pharmaceutical care was shown to improve HRQoL. A meta-analysis of education interventions in COPD did not find consistently effectiveness in HRQoL (27). Our study illustrated that significant improvements over the control group in impact and symptoms scores of SGRQ were achieved at 6-month pharmaceutical care.
Our findings highlight for the first time the significance of pharmaceutical care for medication adherence in non-compliant patients with COPD. We report beneficial effects of medication adherence on reducing hospitalization and enhancing HRQoL. To confirm the generalizability of our findings, a multi-centre prospective randomized controlled study is warranted in large samples of COPD patients from other geographical areas.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007;370:765-73. [PubMed]
- Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 2011;139:752-63. [PubMed]
- Grouse L. The rise of a non-communicable disease epidemic. J Thorac Dis 2012;4:238-9. [PubMed]
- Bryant J, McDonald VM, Boyes A, et al. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir Res 2013;14:109. [PubMed]
- Vestbo J, Anderson JA, Calverley PM, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax 2009;64:939-43. [PubMed]
- Antoniu SA. Adherence to inhaled therapy in COPD: effects on survival and exacerbations. Expert Rev Pharmacoecon Outcomes Res 2010;10:115-7. [PubMed]
- Simpson RJ Jr. Challenges for improving medication adherence. JAMA 2006;296:2614-6. [PubMed]
- Khdour MR, Kidney JC, Smyth BM, et al. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009;68:588-98. [PubMed]
- Jarab AS, Alqudah SG, Khdour M, et al. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 2012;34:53-62. [PubMed]
- Garcia-Aymerich J, Hernandez C, Alonso A, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respir Med 2007;101:1462-9. [PubMed]
- Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? Am J Respir Crit Care Med 1999;160:2000-5. [PubMed]
- Solomon DK, Portner TS, Bass GE, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38:574-85. [PubMed]
- Schlenk EA, Bernardo LM, Organist LA, et al. Optimizing Medication Adherence in Older Patients: A Systematic Review. J Clin Outcomes Manag 2008;15:595-606. [PubMed]
- Wu JY, Leung WY, Chang S, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ 2006;333:522. [PubMed]
- Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006;296:2563-71. [PubMed]
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786-96. [PubMed]
- Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;163:585-91. [PubMed]
- Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax 2001;56:880-7. [PubMed]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. [PubMed]
- Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028-35. [PubMed]
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487-97. [PubMed]
- Turner BJ, Hecht FM. Improving on a coin toss to predict patient adherence to medications. Ann Intern Med 2001;134:1004-6. [PubMed]
- George J, Kong DC, Thoman R, et al. Factors associated with medication nonadherence in patients with COPD. Chest 2005;128:3198-204. [PubMed]
- Rand CS. Patient adherence with COPD therapy. Eur Respir Rev 2005;14:97-101.
- Marteau TM, Hollands GJ, Fletcher PC. Changing human behavior to prevent disease: the importance of targeting automatic processes. Science 2012;337:1492-5. [PubMed]
- Rees PJ. A disease-specific self-management program reduced hospital utilization and improved health status in COPD. ACP J Club 2003;139:65. [PubMed]
- Monninkhof E, van der Valk P, van der Palen J, et al. Self-management education for patients with chronic obstructive pulmonary disease: a systematic review. Thorax 2003;58:394-8. [PubMed]