Early oral feeding following esophagectomy
Introduction
Esophageal cancer is among the fastest growing human cancers and causes more than 400,000 deaths worldwide annually (1). Currently, esophagectomy is still the standard treatment for patients with resectable esophageal cancer. However, esophagectomy which usually involves three compartments (neck, thorax and abdomen) of the body, is considered to be one of the most traumatic operations (2,3). To reduce the surgical trauma of the traditional open esophagectomy, recently minimally invasive esophagectomy (MIE) is broadly applied in clinical practice (4). However, although a lot of improvements in the surgical techniques for esophagectomy have been achieved, postoperative care does not differ between MIE and open surgery. Nil by mouth is still necessary for patients with MIE.
The role of nutrition in the treatment of esophageal cancer is an important, multifaceted aspect of patient care. Methods of nutritional support have been widely described and include enteral nutrition (EN) and parenteral nutrition (PN). Recently, increasing numbers of surgeons have accepted the concept that EN supply should be the first choice for patients with esophagectomy. There are three possible routes for EN supply following esophagectomy: via early oral feeding (EOF), a nasojejunal tube or a jejunostomy tube. However, there is no consensus about the best feeding route and the proper timing of postoperative oral feeding after esophagectomy. We will review studies on the emerging EOF protocol after esophagectomy.
Enteral feeding after esophagectomy: why?
Traditionally, patients were not fed enterally after gastrointestinal surgery until there was evidence that the ileus had resolved clinically, usually in the form of flatus. However, it is now accepted that enteral nutritional support is safer and more efficacious whenever possible, with data including several studies of early EN (jejunostomy) following major upper GI resection (5). It is of vital importance to use the gastrointestinal tract to achieve a trophic effect and activity in the small intestinal mucosa, something that probably could be achieved only by a small amount of EN. The previous studies assert that as little as 300 mL/day is required to prevent changes in intestinal permeability caused by nil by mouth (6). More and more surgeons accept the concept that “when the gut works use it”.
Aiko et al. (7) compared central line feeding alone and combined jejunostomy with central line feeding. The serum lymphocyte count was lower and C-reactive protein and serum bilirubin levels were higher in the combined group. No other statistically significant differences were found in nutritional status or complications at 7 days between the two groups. The authors conclude that parental nutrition leads to a reduced lymphocyte count and raised C-reactive protein and bilirubin levels, suggesting that, biochemically at least, EN has some benefit. Similarly, Gabor et al. (8) compared central line feeding alone with a combined nutrition supply (jejunostomy with central line feeding) in a case-control study. Overall hospital stay and intensive care were shorter in the combined nutrition supply (jejunostomy with central line feeding) group. However, in this study the rate of anastomotic leakage is extremely high (52% for PN alone and 48% for combined routes). A study from Kobayashi et al. (9) showed that early EN started within 3 days is safe and valid for postoperative esophageal cancer patients and has advantages in reducing the use of albumin infusion and PN, for promoting early recovery of intestinal movement, and for early recovery from systemic inflammation.
At present, there is no consensus about the length of the placement of an enteral feeding tube. Several centers have reported on the value of long-term home enteral feeding in some patients with esophagectomy (10,11). The results from a randomized controlled trial of standard care versus six weeks of home EN after esophagectomy or gastrectomy for cancer showed that home enteral feeding by jejunostomy was feasible, safe and acceptable to patients and their caregivers (12). However, the investigators concluded that whether home enteral feeding should be considered as a routine practice would require further confirmation in an appropriately powered, multicenter study (12). Presumed benefits of long-term jejunostomy tube feeding are a more rapid functional recovery and the reduction of weight loss. However, the previous study showed that although the routine placement of jejunostomy tube feeding following esophagectomy, in most patients’ significant weight loss is observed at 6 months postoperatively (13). This may be partly explained by the tremendous surgical trauma of the esophagectomy, which inevitably leads to significant weight loss (14), partly by the difficulty to meet required oral nutritional supply after esophagectomy. At present, the practice of routine long-term home enteral tube feeding after esophagectomy has not been established.
EOF after esophagectomy: why not?
Although tube-feeding after esophagectomy is widely accepted, tube-feeding is not a perfect method for nutritional supply after esophagectomy. A previous study showed that dependence on tube feeding may lead to adverse swallowing ability recovery, potentially due to decreased use of swallowing musculature (15). A review from Weijs et al. (16) showed that the mortality rate associated with surgical placement of a jejunostomy feeding tube during esophagectomy is 0–0.5% and the reoperation rate associated with jejunostomy feeding tube placement is 0–2.9%. There are some minor complications associated with jejunostomy feeding tube placement occur frequently, including gastrointestinal tract complaints (10–39%), entry site leakage (1.4–25%) and entry site infection (0.4–16%). A feeding tube dislocation occurring in 20–35% of all patients and a reduced quality of life (QOL) is the main drawback of using nasojejunal tubes after esophagectomy.
In addition, the emotional, psychological, and physical consequences of living with a feeding jejunostomy tube and the associated feeding are unknown, from both the caregiver and patient perspectives. The majority of patients with tube feeding complain of distress regarding the gustatory deprivation experiences related to drinking liquids, chewing, tasting and swallowing food, exposure to unsatisfied appetites for certain food and forbidden foods, the experiences of dry mouth and thirst (17). In addition, there is the suffering related to the deprivation of social activities usually associated with having food together with friends and relatives (18).
No doubt that oral feeding should be the first choice whenever it is safe and feasible after surgery. Saliva is normally produced when eating and keeps the mouth clean. However, saliva production is often reduced during nutritional support, and the oral mucosa can develop sores. Feeding tubes may reduce salivary flow and subsequently change oropharyngeal colonization in patients with tube feeding. Increased incidence of oropharyngeal colonization with respiratory pathogens is also caused by impairment of salivary clearance (17). In patients with esophagectomy, why must we keep patients’ nil by mouth and choose enteral tube feeding? There are some worries concerning immediate oral feeding after esophagectomy. First, immediate oral feeding after esophagectomy may result in gastric emptying problems. Second, immediate oral feeding after esophagectomy may result in aspiration, especially for patients with recurrent laryngeal nerve (RNL) injury. Third, immediate oral feeding after esophagectomy may increase the risk of anastomotic failure. However, none of these worries have clinical evidence.
EOF after esophagectomy: where we are?
At present, there is no consensus about when to initiate oral feeding and what type of food to try first in patients with esophagectomy. In 2008, the results of an RCT showed that on the first day after major upper GI surgery allowing patients to have regular food at will did not increase postoperative morbidity compared with traditional enteral tube feeding and nil by mouth (19). However, in this randomized study only 8 patients with esophagectomy were enrolled including 6 transthoracic esophagectomies and 2 transhiatal esophagectomies. Subgroup analysis could not be performed due to the small sample size.
At present, there are only 4 studies that have tried to investigate the feasibility and safety of EOF after esophagectomy (Table 1). A prospective multicenter nonrandomized clinical trial from the Netherlands showed that immediate postoperative oral nutrition did not increase the pneumonia rate (28% in EOF group, 40% in the LOF group, P=0.202) and the anastomotic leakage (14% in EOF group, 24% in the LOF group, P=0.202) (23). The 90-day mortality rate was the same between the two groups (2%). Intensive care unit stay and hospital stay were significantly shorter in patients with the immediate oral intake protocol (23). The authors concluded that the immediate initiation of oral feeding after esophagectomy seems to be feasible and does not increase postoperative complications compared to a historical cohort and the literature. However, in this study, the median caloric intake at POD 5 in the EOF group was 58% of what was required. In addition, 38% of the patients needed the nonoral nutrition (23). In addition, this study only included patients who underwent Ivor-Lewis esophagectomy. The RCT study by Mahmoodzadeh et al. (20) showed that EOF after the surgical resection of gastric and esophageal tumors is safe and is associated with better early in-hospital outcomes and a shorter recovery of gastrointestinal function and hospital stay. However, this study included not only patients with esophagectomy but also patients with gastrectomy. In addition, this study had a high risk for bias since patients with complications were excluded.

Full table
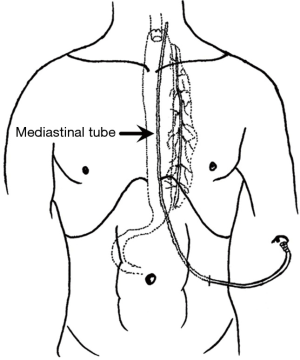
In 2015, our pilot study showed that postoperative gastric emptying is faster than preoperative gastric emptying and that EOF in patients with thoracolaparoscopic esophagectomy is feasible and safe (22). After that, we designed and initiated an RCT for an EOF protocol after MIE. For patients in the EOF group, there was no nasogastric tube, no J-tube or nasoenteral feeding tube, and we only placed one mediastinal drainage tube during surgery (Figure 1). The results of our RCT showed that for patients with McKeown MIE EOF is noninferior to the standard of nil by mouth care with regard to postoperative cardiac, respiratory, and gastrointestinal (CRG) complications (30.0% in the EOF group vs. 32.9% in the LOF group; 95% CI of the difference: −13.8% to 8.0%). In addition, patients with EOF protocol had a quicker recovery of bowel function and improved short-term QOL (21). Based on the RCT, we further investigated the impact of the EOF protocol on inflammatory cytokines [IL-6, IL-8, TNF-α and monocyte chemotactic protein-1 (MCP-1)] after esophagectomy. The results showed that compared with conventional rehabilitation programs, the EOF protocol may decrease the stress response after McKeown MIE (24). At present, this is the only RCT investigating EOF protocol after esophagectomy. However, this study was a single-center study, and in this study, we only included patients with Mckewon esophagectomy and hand-sewn embedded cervical anastomosis (25).
EOF after esophagectomy: future directions
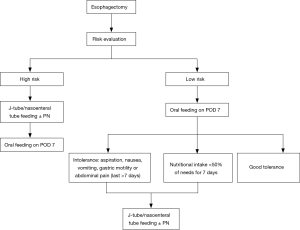
In the future, we will require more multicenter RCTs to investigate the feasibility and safety of the EOF protocol in patients with esophagectomy. In clinical practice, we should select patients who may benefit from EOF, and the clinical decision algorithm suggested by us is shown in Figure 2. A risk model should be built to exclude patients with high risk for an EOF protocol. Furthermore, there are some issues that should be solved in future studies.
Frist, a team including otolaryngology or speech therapy specialists should be included to analyze swallowing deficiencies and assess the patient’s risk of aspiration to with a clinical swallowing examination. Swallow service specialists make recommendations according to the appropriate consistency of foods and liquids, along with other recommendations for swallowing maneuver, such as tucking the chin or turning the head, as well as swallowing exercises to improve the patient’s ability to drink and eat.
Second, further studies are needed to investigate how to improve the amount of oral intake after esophagectomy. Compliance with the recommended daily number of calories was a struggle for most patients with esophagectomy. The amount of oral intake at the time of discharge from hospital is often less than the required nutrition for at least 6 months after surgery in most patients (26). The results from previous studies investigating EOF after esophagectomy showed that most patients could not meet the required amount of kcal when discharged home. In the future, we should investigate how to improve the amount of oral intake after esophagectomy. The dietitian should provide each patient with nutritional supply guidelines to make sure that patients can intake adequate calories and proteins and a list of suggested high-protein and high-caloric nutritional supplements from which to choose, taking into consideration of early satiety, nausea, vomiting, and taste alterations, which are common symptoms observed in patients with esophagectomy.
Third, nutritional markers should be investigated for EOF after esophagectomy. However, there is no ideal single biochemical marker for malnutrition testing, as most laboratory biochemical markers are limited by being not very specific and not sensitive or being affected by other non-nutritional factors. Serum albumin is a protein with liver synthesis and a relatively long half-life and it is one of the most commonly used nutritional markers of nutritional status and prognosis in the absence of overt inflammation. Prealbumin, the protein synthesized by the liver but having a shorter half-life than albumin, could help us monitor short-term nutritional status changes. Subjective and objective data should be collected for a comprehensive nutritional assessment.
Conclusions
In conclusion, evidence supporting an optimal route for nutritional supply in post-esophagectomy patients is weak, and the evidence supporting EOF after esophagectomy is still lacking. In the future, further research studies investigating the safety and efficacy of EOF after esophagectomy are needed, and multidisciplinary teams, including surgeons, nutritionists and nurses, are needed to provide better care to patients with esophagectomy.
Acknowledgements
The authors thank Dr. Misha D. P. Luyer and Dr. Miguel A. Cuesta for inviting us to write this review.
Funding: This study was funded by the Science Foundation for Young Scholars of Henan Cancer Hospital and the Project of Science and Technology of Henan Province (No. 201503185).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Annals of surgery 2009;250:798-807. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg 2017;265:1152-7. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Lewis SJ, Egger M, Sylvester PA, et al. Early enteral feeding versus "nil by mouth" after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ 2001;323:773-6. [Crossref] [PubMed]
- Elia M, Goren A, Behrens R, et al. Effect of total starvation and very low calorie diets on intestinal permeability in man. Clin Sci (Lond) 1987;73:205-10. [Crossref] [PubMed]
- Aiko S, Yoshizumi Y, Sugiura Y, et al. Beneficial effects of immediate enteral nutrition after esophageal cancer surgery. Surg Today 2001;31:971-8. [Crossref] [PubMed]
- Gabor S, Renner H, Matzi V, et al. Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br J Nutr 2005;93:509-13. [Crossref] [PubMed]
- Kobayashi K, Koyama Y, Kosugi S, et al. Is early enteral nutrition better for postoperative course in esophageal cancer patients? Nutrients 2013;5:3461-9. [Crossref] [PubMed]
- Martin L, Lagergren P. Long-term weight change after oesophageal cancer surgery. Br J Surg 2009;96:1308-14. [Crossref] [PubMed]
- Tomaszek SC, Cassivi SD, Allen MS, et al. An alternative postoperative pathway reduces length of hospitalisation following oesophagectomy. Eur J Cardiothorac Surg 2010;37:807-13. [Crossref] [PubMed]
- Bowrey DJ, Baker M, Halliday V, et al. A randomised controlled trial of six weeks of home enteral nutrition versus standard care after oesophagectomy or total gastrectomy for cancer: report on a pilot and feasibility study. Trials 2015;16:531. [Crossref] [PubMed]
- Couper G. Jejunostomy after oesophagectomy: a review of evidence and current practice. Proc Nutr Soc 2011;70:316-20. [Crossref] [PubMed]
- Sakurai Y. Response to nutritional support and therapeutic approaches of amino acid and protein metabolism in surgical patients. J Gastroenterol Hepatol 2013;28 Suppl 4:123-30. [Crossref] [PubMed]
- Langmore S, Krisciunas GP, Miloro KV, et al. Does PEG use cause dysphagia in head and neck cancer patients? Dysphagia 2012;27:251-9. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr 2015;34:1-6. [Crossref] [PubMed]
- Padilla GV, Grant MM. Psychosocial aspects of artificial feeding. Cancer 1985;55:301-4. [Crossref] [PubMed]
- Gibbs-Ward AJ, Keller HH. Mealtimes as active processes in long-term care facilities. Can J Diet Pract Res 2005;66:5-11. [Crossref] [PubMed]
- Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008;247:721-9. [Crossref] [PubMed]
- Mahmoodzadeh H, Shoar S, Sirati F, et al. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today 2015;45:203-8. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann Surg 2018;267:435-42. [Crossref] [PubMed]
- Sun HB, Liu XB, Zhang RX, et al. Early oral feeding following thoracolaparoscopic oesophagectomy for oesophageal cancer. Eur J Cardiothorac Surg 2015;47:227-33. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate Postoperative Oral Nutrition Following Esophagectomy: A Multicenter Clinical Trial. Ann Thorac Surg 2016;102:1141-8. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. The Impact of An Early Oral Feeding Protocol on Inflammatory Cytokine Changes after Esophagectomy. Ann Thorac Surg 2019;107:912-20. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Embedded Three-Layer Esophagogastric Anastomosis Reduces Morbidity and Improves Short-Term Outcomes After Esophagectomy for Cancer. Ann Thorac Surg 2016;101:1131-8. [Crossref] [PubMed]
- Haverkort EB, Binnekade JM, de Haan RJ, et al. Suboptimal intake of nutrients after esophagectomy with gastric tube reconstruction. J Acad Nutr Diet 2012;112:1080-7. [Crossref] [PubMed]