Factors affecting surgical resection and treatment outcomes in patients with pulmonary mucormycosis
Introduction
Mucormycosis is a fungal infection caused by Mucorales species (1). Pulmonary infection is the second most common form of mucormycosis (2), typically affecting severely immunocompromised hosts including patients with hematological malignancies and recipients of hematopoietic stem cell and organ transplants (1,3,4). Pulmonary mucormycosis has emerged as an increasingly relevant and highly lethal cause of invasive fungal infection in many centers worldwide (5-7). However, due to its rarity, the natural course of this disease has not been well evaluated. Pulmonary mucormycosis is difficult to diagnose, and its management is complicated by an aggressive disease course and lack of data to guide treatment decisions (2). Although there has been improvement in the management and outcome of pulmonary mucormycosis over the past few decades, the mortality rate remains high (8,9).
Previous studies have shown that surgical resection is the cornerstone treatment for pulmonary mucormycosis (3,10,11). However, some researchers have raised concerns regarding selection bias in these studies since the patients who underwent surgical resection may have had better overall conditions, resulting in better outcomes. Despite the low level of evidence based on patient data from case reports or other literature, it is difficult to perform a randomized controlled trial (surgical resection versus medical treatment) due to the rarity and aggressive progression of this disease. Based on favorable treatment outcomes in patients who received surgical treatment, surgical resection remains the treatment of choice for pulmonary mucormycosis. However, considering the serious comorbid conditions, some patients cannot undergo surgical resection. There have been few studies comprehensively evaluating the diagnostic and treatment flow in real clinical settings, which could provide information regarding factors that affect the decision to perform surgery.
Based on previous reports and guidelines, we have considered surgery as the first-line strategy for the treatment of pulmonary mucormycosis in our institution since 1996. To evaluate factors affecting the surgical decision and corresponding treatment outcomes of this disease, we performed a retrospective review of patients with pulmonary mucormycosis.
Methods
Study population
This study included patients with pulmonary mucormycosis diagnosed at Samsung Medical Center (a 1,979-bed referral hospital in Seoul, South Korea) from October 1996 to July 2016. The diagnosis of pulmonary mucormycosis was based on histopathological demonstration of tissue invasion by the characteristic hyphae (12), which was compatible with proven cases from the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) (13). Tissue specimens were obtained from surgical biopsy, bronchoscopic biopsy, or computed tomography (CT)-guided percutaneous lung biopsy. This retrospective study was approved by the institutional review board (IRB) of Samsung Medical Center (IRB application No. 2016-10-066). As patient information was anonymized and de-identified prior to analysis, the need for informed consent was waived.
Study design
The patients’ medical records were reviewed, and clinical data were extracted, including presenting symptoms, comorbidities, CT findings, laboratory findings [white blood cell (WBC) count, absolute neutrophil count (ANC), C-reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH)], immunosuppressant medications (steroid, tacrolimus, everolimus, cyclosporine, and mycophenolate mofetil), combined bacterial or viral infections, sequential organ failure assessment (SOFA) score, mechanical ventilation (MV) [including partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio; partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) (PF) ratio], and treatment modalities (including antifungal agents and lung resection surgery). We calculated the SOFA score based on clinical data at the time of pathological disease confirmation. Steroid dose was converted to prednisolone-equivalent dose. Cytomegalovirus (CMV) infection was assessed by the CMV antigenemia assay (14) or CMV culture of bronchoalveolar lavage fluid (15). We defined a nodule as a round opacity measuring up to 3 cm in diameter and a mass as an opacity greater than 3 cm in diameter (without regard to contour, border, or density characteristics) on chest CT (16). Pleural effusion was considered to be associated with pulmonary mucormycosis when present on the same side as lung parenchymal lesions and without other probable cause of pleural effusion. Additionally, the halo sign was defined as a ground-glass opacity (GGO) surrounding a nodule or mass, and the reversed halo sign was defined as a focal rounded area of GGO surrounded by a complete ring of consolidation (16). We evaluated the reasons for not performing surgical resection by chart review. The response to treatment was based on the EORTC/MSG consensus criteria, which incorporate clinical and radiological features (17).
Treatment strategies for pulmonary mucormycosis
During the study period, the general treatment strategies for pulmonary mucormycosis in our institution were as follows. Pulmonary mucormycosis was managed by a multidisciplinary team composed of pulmonologists, intensivists, infectious disease specialists, and thoracic surgeons. Surgical resection was generally recommended as first-line treatment if there were no definite contraindications. An intravenous antifungal agent was initiated immediately after diagnosis of pulmonary mucormycosis. Before August 2015, amphotericin B deoxycholate was used initially, and liposomal amphotericin B was used for patients who developed amphotericin B deoxycholate-associated toxicities. From August 2015, liposomal amphotericin B was used from the beginning of treatment. The optimal duration of antifungal agents was determined at the discretion of attending physicians.
Statistical analyses
Data are presented as median and interquartile range (IQR) for continuous variables and as frequency and percentage for categorical variables. Data were compared with the Mann-Whitney U-test for non-normally distributed continuous variables and with Pearson’s chi-square test or Fisher’s exact test for categorical variables. To explore clinical factors independently associated with a favorable treatment outcome of pulmonary mucormycosis, we fitted a multivariable logistic regression analysis with the stepwise selection method using the minimum Akaike information criterion. Initial variables included in the multivariable logistic regression model were significant at P<0.10 in univariable analyses and included age, body mass index (BMI), and clinical relevance (combined viral infection, longest diameter on chest CT, and lung resection surgery). Firth’s bias-reduced penalized-likelihood method was used in univariable and multivariable logistic regression analyses to reduce bias due to sparsity of the data (18). All tests were two-sided, and P<0.05 was considered significant. Data were analyzed using SPSS for Windows (ver. 23.0; IBM Corp., Armonk, NY, USA) and the ‘logistf’ package in R 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
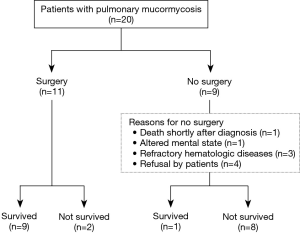
During the study period, a total of 20 patients were diagnosed with pulmonary mucormycosis. For six of the patients, some clinical data were included in a recently published article (19); data on the remaining patients have not been previously reported. Of the 20 patients, 9 (45%) did not undergo pulmonary resection surgery. The reasons for not undergoing surgery were death shortly after diagnosis (n=1), altered mental state (n=1), refractory underlying hematologic disease (n=3), and refusal by patient due to concern of operative risk (n=4).
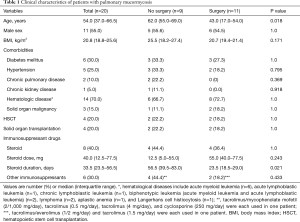
Clinical characteristics of the patients are summarized in Table 1. The median age was 54 years (IQR, 37.0–66.5 years), and 11 patients were male (55%). Six patients (30%) had diabetes mellitus and 14 (70%) had hematological diseases. Four patients (20%) received hematopoietic stem cell transplantation (HSCT) and 4 (20%) received solid organ transplantation, including kidney (n=1) and liver (n=3) transplantation. Eight patients (40%) had received steroids, with a median of 40 mg (IQR, 12.5–77.5 mg) for a median of 33.5 days (IQR, 23.5–56.5 days).
Full table
Compared with the patients who underwent surgical resection, the patients who did not were more likely to be older (median 62 vs. 43 years; P=0.018) and have a longer duration of steroid use (median 56.5 vs. 23.5 days; P=0.021). However, there was no significant difference in sex or comorbidities, including diabetes mellitus, hypertension, chronic pulmonary disease, chronic kidney disease, hematologic malignancy, and solid organ malignancy. In addition, there was no difference in proportion of patients who underwent HSCT or solid organ transplantation.
Clinical, laboratory, and radiological findings in patients with pulmonary mucormycosis
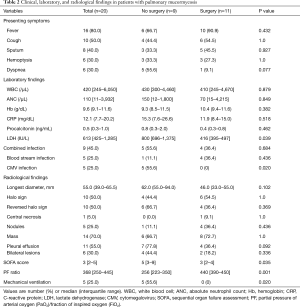
Fever (80%, n=16) was the most common clinical manifestation, followed by cough (50%, n=10), sputum (40%, n=8), and hemoptysis (30%, n=6). There were no significant differences in presenting symptoms; laboratory findings including WBC, hemoglobin, CRP, and procalcitonin; combined bacterial infection; or radiologic findings between the patients who underwent surgical resection and those who did not. However, patients who did not undergo surgical resection had significantly higher LDH level [median 800 IU/L (IQR, 696–1,375 IU/L) vs. 416 IU/L (IQR, 395–497 IU/L); P=0.039) and SOFA score [median 5 (IQR, 3–9) vs. 3 (IQR, 2–4), P=0.035] and significantly lower PF ratio [median 256 (IQR, 223–350) vs. median 440 (IQR, 390–450), P=0.001) compared to patients who underwent surgical resection. In addition, the proportions of patients with CMV infection [55.6% (5/9) vs. 0% (0/11); P=0.020) and receiving MV [55.6% (5/9) vs. 0% (0/11); P=0.020] were also higher in patients who did not undergo surgery. Regarding radiological findings, patients who did not undergo surgical resection were more likely to have larger pulmonary lesions [median 62.0 mm (IQR, 55.0–94.0 mm) vs. 46.0 mm (33.0–55.0 mm); P=0.102] and mucormycosis-associated pleural effusion [77.8% (7/9) vs. 36.4% (4/11); P=0.092] on chest CT compared to patients who underwent surgical resection (Table 2). However, these differences were not statistically significant.
Full table
Treatment modalities and outcomes
Of the 20 patients with pulmonary mucormycosis, 10 (50%) survived. Among the nine patients who did not undergo surgical resection, only 1 (11%) survived, and the other 8 (89%) died within 4 weeks after initial diagnosis due to pulmonary mucormycosis. In contrast, of the 11 patients who underwent pulmonary resection surgery, 9 (82%) survived and 2 (18%) died from ventilator-associated pneumonia on day 25 and complicated surgical wound infection on day 61 after initial diagnosis, respectively (Figure 1).
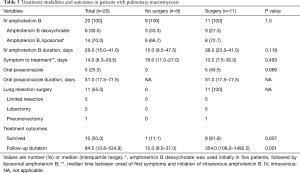
Regarding antifungal treatment, amphotericin B deoxycholate was prescribed in 6 patients (30%), liposomal amphotericin B in 9 (45%), and amphotericin B deoxycholate followed by liposomal amphotericin B in 5 (25%). Median time between onset of first symptoms and initiation of amphotericin B was 14.0 days (IQR, 8.3–29.5 days). There was no significant difference in antifungal treatment between patients who underwent surgery and those who did not (Table 3).
Full table
Comparison of clinical characteristics and treatment outcomes between patients who refused surgery and those who underwent surgery
Baseline characteristics and treatment outcomes in patients who refused surgery and those who underwent surgery are summarized in Table S1. There were no significant differences in sex, blood stream infection, and radiological findings despite relatively prolonged steroid use in patients who refused surgery compared to those who underwent surgery (median 43 vs. 24 days, P=0.034). There were no patients who had CMV infection and who received prolonged use of MV (≥3 days) in either patient group. Although the patients who refused surgery were more likely to be older and have a higher SOFA score, these differences were not statistically significant (P=0.050 for age, P=0.054 for SOFA score). Regarding treatment outcomes, survival rate was significantly higher in patients who underwent surgery compared to those who refused surgery [25.0% (1/4) vs. 90.9% (10/11), P=0.033].
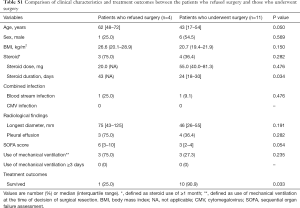
Full table
Clinical factors affecting survival of patients with pulmonary mucormycosis
As shown in Table 4, pulmonary resection surgery was the only clinical factor associated with survival in both univariate [odds ratio (OR) for survival =21.53, 95% confidence interval (CI), 2.08–223.04; P=0.010) and multivariable analyses (adjusted OR for survival =15.45, 95% CI, 1.33–179.28; P=0.029). Although age, BMI, viral infection, and longest diameter on CT were marginally significantly associated with treatment outcomes of pulmonary mucormycosis, these factors were not significant in multivariable analyses.
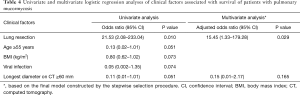
Full table
Discussion
In this study, we showed that about half of the patients with pulmonary mucormycosis did not undergo surgical resection mainly due to underlying disease and the concern of operative risk. Unfortunately, most patients who did not undergo surgery died within 1 month of the initial diagnosis. The other half of the patients underwent surgical resection, and about 80% of these patients survived. These findings clearly indicate that surgical resection should continue to be the mainstay of treatment to overcome the serious disease burden of pulmonary mucormycosis.
Since mucormycosis is a highly angioinvasive infection with extensive thrombosis and tissue necrosis (20), antifungal agents may have poor penetration at the site of infection and have a limited effect on successful treatment without surgical resection. Overall survival rates in previous studies were 46%, 45%, and 35%, but surgically treated patients demonstrated much higher survival rates of 51%, 73%, and 90%, respectively (3,10,11). Consistent with previous studies, the survival rate in our study was significantly higher in patients who received pulmonary resection surgery (82%) than in those who did not (11%). The strength of our study is that it was conducted in a single cohort, in contrast to previous studies that extracted patient data from other studies (3,10,11).
Despite the importance of surgical resection, some patients cannot undergo surgery, most likely due to serious comorbid conditions and/or concerns for operative risks. However, few studies have comprehensively evaluated the diagnostic flow in real clinical settings. Our study overcame this limitation and provided the reasons for not undergoing surgical resection in real clinical practice. In our study, one patient with altered mental state and three patients with refractory hematologic disease did not receive surgical resection because surgery might not have changed their overall clinical course; brain MRI performed to the patient with altered mental state revealed multiple embolic infarction, which may have been caused by fungal infection. There were also several signs such as persistent dilatation of pupils and EEG findings suggestive of irreversible brain damage. Therefore, our multidisciplinary team decided not to perform surgery. However, an additional four patients refused surgery due to the concern for operative risk even though their underlying diseases were not progressive or refractory. In such cases, real-world clinic data showing extremely poor treatment outcomes in patients not undergoing surgical resection may have persuaded the patients to undergo surgical resection while confronting the operative risk. Together with the findings of previous studies (3,10,11), our results strongly suggest that clinicians should not delay pulmonary resection despite operative risks, especially in patients with stable underlying disease.
In this study, the overall survival rate of patients with pulmonary mucormycosis was 50%, which was similar to or slightly higher than the rate of previous studies (30–50%) (10,11). The explanation for this might be that our patients received effective treatment following a timely diagnosis, according to the recommendations of recent clinical guidelines (13). We initiated effective intravenous antifungal agents when pulmonary mucormycosis was suspected, even before confirmative diagnosis. In agreement with our findings, Chamilos et al. reported that initiating antifungals within 5 days after diagnosis of mucormycosis was associated with improved survival compared to initiating treatment 6 days after diagnosis (21).
In addition to surgery and timely treatment, the underlying condition of the patients may have influenced the treatment outcomes. Lewis et al. reported that a higher LDH level at the time of diagnosis is an independent marker for rapid disease progression and death in patients with pulmonary mucormycosis (22). In our study, non-surviving patients had significantly higher LDH level than surviving patients, which probably reflects the activity of underlying hematological or malignant diseases. Furthermore, non-survivors might be more immunocompromised compared to survivors, as concomitant CMV infections were observed only in non-survivors. Thus, successful treatment of pulmonary mucormycosis also relies on reversing underlying predisposing factors.
Pulmonary mucormycosis often demonstrates non-specific radiological findings, including infiltration, consolidation, nodules, and effusion on chest CT (23). Although one study insisted that the reversed halo sign is useful to distinguish pulmonary mucormycosis from other invasive fungal infections (24), no pathognomonic CT feature was observed in our study, similar to the findings of other reports (10,25). Chamilos et al. reported that presence of pleural effusion on initial CT scan is an independent predictor for poor treatment outcome in patients with pulmonary mucormycosis (21). However, in our study, pleural effusion was not associated with poor treatment outcomes despite a tendency of higher mortality among patients with pleural effusion over those without pleural effusion. Regarding this issue, subsequent studies with a larger number of patients are needed.
Our study also provides very informative data that longer diameter on CT scan, which might reflect the extent of pulmonary mucormycosis, is associated with a poor clinical outcome. Among subjects who survived, one who did not undergo surgery had a relatively small lesion (38 mm of maximal diameter on CT). Fortunately, this patient did not receive MV support and had no concurrent bacteremia or viral infection. Although our study suggests that surgical resection is associated with better clinical outcomes, this case indicates that patients with small lesions might be treated successfully with appropriate antifungal drugs.
Our study had several limitations. First, it was a retrospective study conducted at a single referral center in South Korea. Second, the low survival rate might have been affected by multiple factors other than surgery such as MV support, CMV infection, and organ failures though surgery seemed the most important factor to improve survival in patients with pulmonary mucormycosis. Third, we did not evaluate whether survival differed according to Mucorales species. Since the diagnosis of mucormycosis was based on histopathologic findings, information on the species was not available. Fourth, due to the relatively small sample size, it was difficult to demonstrate statistical significance for some of the factors affecting treatment outcomes of pulmonary mucormycosis.
In conclusion, our study showed that about half of the patients with pulmonary mucormycosis underwent surgical resection. The main reasons for not undergoing surgery were the gravity of underlying diseases and concerns of operative risk. Although overall survival rate of patients with pulmonary mucormycosis was poor, it was significantly higher in those who received pulmonary resection surgery. Therefore, a timely decision to undergo lung resection might be crucial to improve survival in patients with pulmonary mucormycosis, and more surgical-oriented treatment strategies need to be considered in such patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the institutional review board (IRB) of Samsung Medical Center (IRB application No. 2016-10-066).
References
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000;13:236-301. [Crossref] [PubMed]
- Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013;98:492-504. [Crossref] [PubMed]
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634-53. [Crossref] [PubMed]
- Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005;18:556-69. [Crossref] [PubMed]
- Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis 2009;15:1395-401. [Crossref] [PubMed]
- Kume H, Yamazaki T, Abe M, et al. Increase in aspergillosis and severe mycotic infection in patients with leukemia and MDS: comparison of the data from the Annual of the Pathological Autopsy Cases in Japan in 1989, 1993 and 1997. Pathol Int 2003;53:744-50. [Crossref] [PubMed]
- Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 2009;48:265-73. [Crossref] [PubMed]
- Pagano L, Caira M, Candoni A, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica 2006;91:1068-75. [PubMed]
- Torres-Narbona M, Guinea J, Martinez-Alarcon J, et al. Impact of zygomycosis on microbiology workload: a survey study in Spain. J Clin Microbiol 2007;45:2051-3. [Crossref] [PubMed]
- Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med 1999;159:1301-9. [Crossref] [PubMed]
- Tedder M, Spratt JA, Anstadt MP, et al. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg 1994;57:1044-50. [Crossref] [PubMed]
- Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood 2011;118:1216-24. [Crossref] [PubMed]
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- van der Bij W, Schirm J, Torensma R, et al. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J Clin Microbiol 1988;26:2531-5. [PubMed]
- Uberti-Foppa C, Lillo F, Terreni MR, et al. Cytomegalovirus pneumonia in AIDS patients: value of cytomegalovirus culture from BAL fluid and correlation with lung disease. Chest 1998;113:919-23. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis 2008;47:674-83. [Crossref] [PubMed]
- Firth D. Bias Reduction of Maximum Likelihood Estimates. Biometrika 1993;80:27-38. [Crossref]
- Hong Y, Park J. The Role of Transbronchial Lung Biopsy in Diagnosing Pulmonary Mucormycosis in a Critical Care Unit. Korean J Crit Care Med 2017;32:205-10. [Crossref]
- Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med 2011;32:693-702. [Crossref] [PubMed]
- Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008;47:503-9. [Crossref] [PubMed]
- Lewis RE, Georgiadou SP, Sampsonas F, et al. Risk factors for early mortality in haematological malignancy patients with pulmonary mucormycosis. Mycoses 2014;57:49-55. [Crossref] [PubMed]
- Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7-14. [Crossref] [PubMed]
- Wahba H, Truong MT, Lei X, et al. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis 2008;46:1733-7. [Crossref] [PubMed]
- McAdams HP, Rosado de Christenson M, Strollo DC, et al. Pulmonary mucormycosis: radiologic findings in 32 cases. AJR Am J Roentgenol 1997;168:1541-8. [Crossref] [PubMed]