
Exceptional antitumor responses beyond immune checkpoint inhibition in non-small cell lung cancer patients: insights into optimal therapy sequencing
Introduction
Lung cancer represents the leading cause of cancer mortality, with an estimated of 222,500 new cases and 155,870 deaths in the United States in 2017 (1). The majority of patients present with metastatic disease at the time of initial diagnosis, with 5-year survival rates of 2% (2).
Recent data have shown that cancer immunotherapy may represent an efficient therapeutic modality for patients suffering from non-small cell lung cancer (NSCLC). In particular, suppression of the programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) axis has established immense as well as prolonged antitumor responses (3). As a result, immune checkpoint inhibitors targeting the above-mentioned pathway have gained approval for use in both second and first-line treatment of individuals suffering from advanced NSCLC (4). Nevertheless, results on the perfect response-predicting test are still pending (5). In addition, emerging data unveiling potential interplay between immune checkpoint inhibitors and classic chemotherapy have placed proper sequence and combination of different therapies at the center of research interest (6-8).
We herein present a series of five cases of NSCLC patients demonstrating significant responses at salvage chemotherapy following immune checkpoint inhibition, seen at a single institution over a 24-month period and review the current literature on this topic.
Materials and methods
A retrospective chart review of all NSCLC patients diagnosed and treated in Sotiria General Hospital, Athens, Greece, from January 2016 to December 2017 was conducted. All patients were age 18 or greater. Diagnosis of lung cancer was either histologic or cytological. Staging was performed using the 7th edition of the TNM classification for lung cancer. Therapeutic management was based on clinical practice guidelines and further modified on a case-by-case basis. Patient demographics, clinical characteristics, treatments administered, tumor responses per RECIST 1.1 or, whenever applicable, iRECIST criteria and outcomes were assessed.
Results
Among a total of 18 patients with advanced-stage NSCLC who had received salvage chemotherapy after second or subsequent-line immunotherapy, five died before completion of the second chemotherapy cycle, four had progressive disease (PD) and the remaining nine patients had either stable disease (SD; 4/18) or partial response (PR; 5/18), corresponding to an objective response rate (ORR) of 27.8% and a disease control rate (DCR) of 50%. The clinicopathological and treatment features of partial responders were further analyzed. All patients with PR were males. Their median age at diagnosis was 61 years (range, 55–72 years). Adenocarcinoma was the most prevalent lung cancer subtype, seen in 60% of patients. Four out of 5 individuals had metastatic disease at immunotherapy initiation (cases 1, 3, 4 and 5). Four patients received sole PD-1 inhibition and one received a combination of PD-1 and CTLA-4 inhibitors. Response to immunotherapy was assessed using iRECIST criteria in all five cases. SD was the best tumor response achieved during immune checkpoint inhibition and was observed in 80% of patients; one patient (case 5) experienced immediate disease progression. Subsequent responses following immunotherapy were assessed per RECIST 1.1 criteria. Nab-paclitaxel or carboplatin administration followed immune checkpoint blockade in all cases. Tumor-reducing procedures, including palliative radiotherapy or surgery were performed in 4 out of 5 cases (cases 1, 2, 3 and 5). The more salient clinical information on these five cases, including percent tumor reduction beyond immune checkpoint inhibition, are summarized in Table 1. Although a validated Quality of Life (QoL) questionnaire was not used in this study, retrospective analysis of patients’ charts revealed that all responders had direct and significant symptomatic improvement following administration of salvage chemotherapy. All patients provided signed informed consent for publication of medical information and images concerning their cases. Furthermore, any clinical or personal data that could reveal the patient’s identity were removed from the text and images so as to ensure anonymity.

Full table
Case 1
A 63-year-old male was diagnosed with stage IIIA lung adenocarcinoma. A left upper lobectomy was performed, followed by four cycles of adjuvant chemotherapy with cisplatin and vinorelbine. PD occurred 2 months post adjuvant therapy termination, with metastatic dissemination in bones and both adrenal glands. First-line therapy with carboplatin, pemetrexed and bevacizumab was started, in conjunction with palliative bone radiotherapy. Nivolumab at a dose of 3 mg/kg was chosen as second line treatment at disease progression. Palliative bilateral adrenalectomy was also performed. SD was the best tumor response attained during second-line treatment. After 28 2-week cycles, the patient demonstrated clinical disease progression with the appearance of cutaneous nodules. Restaging scans showed recurrent left adrenal lesion, peritoneal carcinomatosis, abdominal soft tissue lesions, enlarged mediastinal and bilateral supraclavicular lymph nodes. Third-line treatment consisted of 6 cycles of nab-paclitaxel and nintedanib. Patient achieved PR, with complete eradication of thoracic lesions, at 4 cycles, maintained for 4 months following treatment termination.
Case 2
A 55-year-old male presented to the outpatient clinic for the evaluation of new onset, nonproductive cough. Chest CT demonstrated a right hilar mass infiltrating trachea and enlarged right hilar and mediastinal lymph nodes. Diagnosis of squamous NSCLC was made by bronchoscopy and endobronchial biopsy. Subsequent imaging evaluation was negative for metastases, so disease was staged as IIIB (T4N2M0) at the time of diagnosis. The patient was treated with concurrent chemoradiation. Five months after the end of first-line treatment, disease progression occurred. Nivolumab at 3 mg/kg was administered as second-line treatment and an endotracheal “Y” stent was placed in the trachea and left main bronchus due to impending obstruction of the left lung due to bronchial infiltration. SD was accomplished after the first 4 cycles of nivolumab. The patient had received 7 cycles of Nivolumab when PD was radiographically confirmed. We proceeded to nab-paclitaxel rescue treatment in concurrence with palliative chest irradiation because of life-threatening hemoptysis. PD was observed once again after 4 nab-paclitaxel cycles, but the patient responded to fourth-line treatment with carboplatin and gemcitabine. PR was established with both primary lesion shrinkage and eradication of hilar and mediastinal lymph nodes on chest CT, as well as an endoscopic improvement indicating the need for stent removal.
Case 3
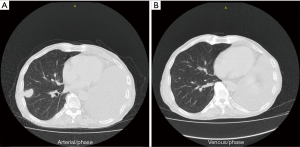
A 72-year-old male patient presented with nonproductive cough and hemoptysis. Chest CT scan exhibited a single mass on his left upper lobe and enlarged mediastinal lymph nodes. Diagnosis of NSCLC not otherwise specified (NOS) was obtained via bronchoscopy. Complete staging showed IIIA disease (T3N2M0). Concurrent chemoradiation was elected for this patient. At his first restaging a new lesion had emerged on his right adrenal gland. Second-line therapy with nivolumab at a dose of 3 mg/kg was administered, with SD designating best tumor response to treatment. The patient underwent left pneumonectomy because of massive hemoptysis. Palliative adrenalectomy was also performed. After 7 cycles of nivolumab, he experienced disease progression with new lesions on his right lung. Third-line treatment with carboplatin and nab-paclitaxel was chosen. After completion of the first 4 cycles, restaging CT scans indicated PR per RECIST 1.1 criteria (Figure 1) (80.4% decrease of target lesions and elimination of non-target lesions) and 2 more cycles were administered. Within the first 2 months after treatment completion, the patient experienced disease flare with doubling of the existing and emergence of multiple new thoracic lesions.
Case 4
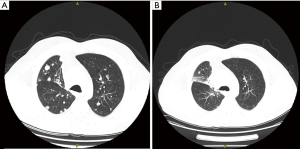
A 60-year-old male was diagnosed with stage IVa lung adenocarcinoma, with no targetable mutations or PD-L1 expression. He was started on first-line therapy with carboplatin, vinorelbine and bevacizumab for 6 cycles. Bevacizumab maintenance followed for another 6 cycles. The patient experienced disease progression with development of a new liver lesion. At that time, the patient agreed to participate in a clinical trial and received 4 cycles of tremelimumab and durvalumab as second-line treatment, plus 6 cycles of durvalumab for maintenance. SD was observed as best response to combined immune checkpoint inhibition, followed by disease progression with development of new lung and liver lesions. Nab-paclitaxel was elected as third-line therapy. After 4 cycles, PR was accomplished with 49% reduction of the patient’s tumor burden (Figure 2). As soon as the 6th cycle was completed, patient presented with focal neurologic deficits, leading to identification of new tumor lesions on his brain MRI.
Case 5
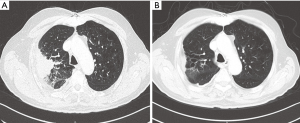
A 61-year-old male patient was diagnosed with EGFR wild-type, ALK negative, stage IV (T2N3M1a) NSCLC adenocarcinoma. After demonstrating disease progression at 3 lines of chemotherapy doublets, the patient was eventually continued on nivolumab at a dose level of 3 mg/kg. Shortly after completion of the fourth nivolumab cycle, he experienced radiographic disease “flare” on the chest and new brain and liver lesions. Carboplatin with vinorelbine and concurrent whole brain radiotherapy (WBRT) were elected. After 4 chemotherapy cycles, PR—per RECIST 1.1—was achieved, with 70% decrease of his primary tumor and elimination of brain and liver metastases (Figure 3).
Discussion
Exceptional antitumor responses, occurring beyond second-line therapy are particularly rare among NSCLC patients. Herein, we present a series of five patients suffering from NSCLC that achieved notable responses at salvage chemotherapy after immunotherapy administration.
Inhibitors of PD-1/PD-L1 axis have originally exhibited substantial benefit at disease progression during or after chemotherapy administration, gaining approval for use in second-line therapy of NSCLC patients (9,10). As reviewed elsewhere, classic chemotherapeutic drugs, paclitaxel and platinum agents included, may carry certain immunomodulatory properties (11). Chemotherapy-induced immunogenic cell death (ICD) represents a prominent pathway towards achieving robust immune responses and, subsequently, long-term therapeutic responses (12,13). Immune cell recruitment to the tumor microenvironment, amplification of both natural-killer and cytotoxic T-cell responses, selective exhaustion of immune suppressive cells, including regulatory T cells and enhancement of antigen presentation turning dying tumor cells into live vaccines are only some of the effects rendering cytotoxic chemotherapy a perfect prelude to subsequent immunotherapy (12,13). Taxanes in particular, have also demonstrated unique immunostimulant potential with the additional upregulation of IL-12, IFN-γ, TNF-α, and GM-CSF (14).
Limited data from phase II clinical trials have also pointed out that phased ipilimumab (two doses of placebo plus paclitaxel/carboplatin followed by four doses of ipilimumab plus paclitaxel/carboplatin) at a dose level of 10 mg/kg performed significantly better in terms of median progression-free survival (PFS) in comparison to concurrent (four doses of ipilimumab plus paclitaxel/carboplatin followed by two doses of placebo plus paclitaxel/carboplatin), or control (up to six doses of placebo plus paclitaxel/carboplatin) regimens, with tolerable toxicity in patients with advanced NSCLC and extensive small cell lung cancer (SCLC) as well (15,16). In melanoma, ipilimumab administration prior to BRAF inhibitor demonstrated a substantial increase in overall survival as compared to subsequent ipilimumab administration after BRAF inhibition, or administration of each agent alone (17).
Recent clinical data have indicated that chemotherapy administered after immune checkpoint inhibitors delivered surprisingly high ORR of 30.9% in NSCLC patients (18). Salvage chemotherapy administered after immunotherapy (SCAI) also achieved significantly higher ORR in comparison with the last chemotherapy administered before immunotherapy (LCBI) (53.4% vs. 34.9%, P=0.03) in patients suffering from NSCLC (19). In addition, ORR to single-agent chemotherapy after immunotherapy exposure in NSCLC patients reached 39%, approaching rates of first-line platinum-based chemotherapy in the same subgroup of patients (20). Individuals treated with PD-1/PD-L1 inhibitors had increased likelihood of accomplishing PR to subsequent chemotherapy in a retrospective study by Leger et al. (21). Furthermore, contemporary analysis from KEYNOTE-024 study, in which cross-over between pembrolizumab and chemotherapy arms was permitted at disease progression, revealed that combined PFS for first-line pembrolizumab followed by subsequent chemotherapy was significantly longer as compared to the PFS of patients receiving first-line chemotherapy followed by second-line pembrolizumab (18.3 vs. 8.4 months, P<0.01) (22).
It seems that PFS is not adequate for illustrating the entire gain of immune checkpoint inhibition in patients with cancer. This fact could provide an acceptable explanation for the inability of PD-L1 status to unconditionally predict response to treatment, revealing apparent discrepancies in PD-L1 testing clinical significance between first- and second-line therapy. Non-responders, or patients acquiring minimal benefit in terms of percent tumor decline per RECIST or iRECIST criteria after immunotherapy infusion, as seen in our series, could still profit from intensification of the effects of subsequent treatments. Immunotherapy induction could also thrive on an intact immune system before chemotherapy utilization.
With respect to other therapeutic modalities, radiotherapy has been shown to represent an important adjunct to immune checkpoint inhibitors, with emerging data delineating the role of the “abscopal effect” (23). Potential interactions between immune suppressing VEGF inhibitors and immune enhancing agents is an additional field of active investigation.
Conclusions
Immunotherapy represents a leap in cancer treatment, altering the prognosis of thousands of patients worldwide. However, many existing or rising questions remain yet to be answered. Among those, cardinal is the role of identifying a marker or a panel of markers to be used as predictors of response and clinical benefit, as well as the role of combination strategies and sequencing of immunotherapy with conventional treatments as chemotherapy, radiotherapy, tyrosine kinase inhibitor (TKIs) and antiangiogenic factors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging Committee. Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Herzberg B, Campo MJ, Gainor JF. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Oncologist 2017;22:81-8. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther 2017;16:2598-608. [Crossref] [PubMed]
- Attili I, Passaro A, Pavan A, et al. Combination immunotherapy strategies in advanced non-small cell lung cancer (NSCLC): Does biological rationale meet clinical needs? Crit Rev Oncol Hematol 2017;119:30-9. [Crossref] [PubMed]
- Atkins MB, Larkin J. Immunotherapy Combined or Sequenced With Targeted Therapy in the Treatment of Solid Tumors: Current Perspectives. J Natl Cancer Inst 2016;108:djv414. [Crossref] [PubMed]
- Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: A new era in cancer immunotherapy. J Intern Med 2018;283:110-20. [Crossref] [PubMed]
- Spigel DR, Reckamp KL, Rizvi NA, et al. A phase III study (CheckMate 017) of Nivo-lumab (NIVO; anti-programmed death-1 [PD-1]) vs Docetaxel(DOC) in previously treated advanced or metastatic squamous(SQ) cell non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:abstr 8009.
- Opdivo (nivolumab) [summary of product characteristics]. New York: Bristol-Myers Squibb; 2016.
- Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014;21:15-25. [Crossref] [PubMed]
- Wong DY, Ong WW, Ang WH. Induction of immunogenic cell death by chemotherapeutic platinum complexes. Angew Chem Int Ed Engl 2015;54:6483-7. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51-72. [Crossref] [PubMed]
- Soliman HH. nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. Onco Targets Ther 2016;10:101-12. [Crossref] [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Ascierto PA, Margolin K. Ipilimumab before BRAF inhibitor treatment may be more beneficial than vice versa for the majority of patients with advanced melanoma. Cancer 2014;120:1617-9. [Crossref] [PubMed]
- Grigg C, Reuland BD, Sacher AG, et al. Clinical outcomes of patients with non-small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. J Clin Oncol 2017;35:abstr 9082.
- Park SE, Lee SH, Ahn JS, et al. Increased Response Rates to Salvage Chemotherapy Administered after PD-1/PD-L1 Inhibitors in Patients with Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:106-11. [Crossref] [PubMed]
- Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017;112:90-5. [Crossref] [PubMed]
- Leger PD, Rothschild S, Castellanos E, et al. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non-small cell lung cancer. J Clin Oncol 2017;35:abstr 9084.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PDL1 tumor proportion score (TPS) ≥50% enrolled in KEYNOTE-024. J Clin Oncol 2017;35:abstr 9000.
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]


