
Additional role of bronchial mucosal biopsy for ciliary structural abnormality in diagnosis of primary ciliary dyskinesia
Introduction
Primary ciliary dyskinesia (PCD) is a rare autosomal-recessive genetic disorder of cilia, with a prevalence of 1 in 10,000–40,000 live births that is equal in men and women (1,2). Recurrent upper and lower respiratory tract infection is common and is caused by impaired muco-ciliary clearance of respiratory tracts. Despite long-standing chronic respiratory symptoms, patients often receive a diagnosis of PCD after having progressive bronchiectasis and deteriorating lung function (3,4). Early recognition and diagnosis are necessary for optimal management.
There is no single gold standard diagnostic test to capture all PCD defects. Recent guidelines for the diagnosis of PCD recommended a combination of clinical presentations and several diagnostic tests, such as nasal nitric oxide (nNO), analysis of ciliary beat frequency and pattern by high-speed video-microscopy analysis (HSVA), transmission electron microscopy (TEM), genotyping, and immunofluorescence (5,6). Among these methods, TEM is a highly specific test with a key role in confirming the diagnosis of PCD. TEM detects ultrastructural defects in cilia, and most cases are due to a lack of dynein arms. Other defects include disorganization of microtubular doublets or loss of the central microtubular pair (7).
Nasal mucosa is generally selected as the biopsy site for obtaining mucosal tissue containing cilia for TEM because of its easy accessibility (8,9). However, investigation of the ultrastructural ciliary arrangement of nasal mucosa in some patients with chronic sinusitis is not possible due to severely impaired ultrastructure (10). Previous reports also showed a low diagnostic rate of TEM with nasal mucosal biopsy for PCD, ranging from 0.15 to 0.35 among patients who were suspected to have PCD by clinical presentations (9,11-15). Bronchial mucosal biopsy for TEM has been recommended with no significant difference of diagnostic yield between nasal mucosa and bronchial mucosa (8,10). However, there is no available study on whether a combination of nasal mucosal biopsy with bronchial mucosal biopsy has increased diagnostic yield for PCD. Therefore, we aimed to assess the diagnostic value of combination of nasal and bronchial mucosal biopsies in the diagnosis of PCD.
Methods
Patients
The electronic medical records of Samsung Medical Center, Seoul, South Korea, from April 1997 to June 2017 were retrospectively reviewed. In this period, 106 patients received nasal or bronchial mucosal biopsies for TEM under suspicion of PCD. They had chronic or recurrent episodes of lower respiratory infection, sinusitis or otitis media without obvious causes. Among them, 10 patients diagnosed with other diseases, such as common variable immunodeficiency, cystic fibrosis, hyper-immunoglobulin E syndrome, or tumor related immunodeficiency, were excluded. A total of 96 patients were finally analyzed.
Patients were divided into three groups based on the site of mucosal biopsy; nasal mucosal biopsy only group (NB group), bronchial mucosal biopsy only group (BB group), and combination of both nasal and bronchial mucosal biopsies group (NBB group). This retrospective study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2018-04-029-001). Informed consent was waived for the use of patient medical data and patient information was anonymized and de-identified prior to analysis.
Radiologic examination
Radiologic examinations performed before mucosal biopsy was reviewed. Bronchiectasis was diagnosed when the following two key imaging findings were present on chest computerized tomography (CT) scan: (I) internal diameter of the bronchus was larger than that of the accompanying vessels and (II) no bronchial tapering was present in the lung periphery (16).
For sinusitis, ostiomeatal unit (OMU) CT scan and nasal endoscopy were assessed. Sinusitis was defined as clinical symptoms and either endoscopic signs or CT changes based on the 2012 European position paper on rhinosinusitis and nasal polyps guidelines (17).
Nasal mucosal biopsy and bronchial mucosal biopsy
Nasal mucosal biopsies were performed using grasping forceps on the inferior turbinate where the mucosa looked healthy. Biopsies were performed in an outpatient clinic or in an operation room during endoscopic sinus surgery for chronic sinusitis. Among the 79 patients who underwent nasal mucosal biopsy (NB group and NBB group), 54 underwent biopsy in an outpatient clinic and 25 patients underwent biopsy in an operation room during endoscopic sinus surgery.
Bronchial mucosal samples were obtained from the main carina, secondary carina, or lobar bronchus using bronchoscopic forceps under sedation in a bronchoscopy suite. In 37 patients who underwent bronchial mucosal biopsy (BB group and NBB group), 5 were biopsied from the main carina, 2 were biopsied from the left second carina, 13 were biopsied from the right second carina, 8 were biopsied from both sides of the second carina and 9 were biopsied from the lobar bronchus.
Interpretation of pathology
Biopsies were immersed in 2.5% glutaraldehyde for prefixation. Fixed samples were rinsed in 0.1 M phosphate buffer solution at pH 7.4, followed by postfixation using 1% osmium tetroxide (OsO4). For dehydration, a 50% to 90% graded series of ethanol was used for 10 minutes and 100% ethanol was used for 30 minutes. To remove residual ethanol, the samples were immersed in propylene oxide for 30 minutes and embedded in epoxy resin, Oken Epok 812 (Okenshoji, Tokyo, Japan). Semi-thin sections were stained with toluidine blue O (0.6 µm thickness) and ultra-thin sections (60 nm thickness) were stained with 7% uranyl acetate and lead nitrate. Sections were examined at a final magnification of ×100,000 by Hitachi 7100 electron microscope (Hitachi Ltd., Tokyo, Japan).
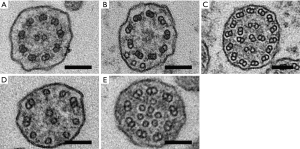
At least 50 transverse ciliary sections were required to satisfy adequacy requirements for evaluation of ultrastructural defects; otherwise, specimens were inadequate for diagnosis (18). Ultrastructural abnormalities in ciliary defects are listed and summarized in Figure 1 and Table 1. Combination of absence or defect in an inner and/or outer dynein arm and abnormalities in number, pair, or location of microtubules are the typical ciliary defects in PCD. These ultrastructural abnormalities are identified in up to 10% of cilia in normal specimens (19,20); therefore, the presence of more than 10% defected cilia was defined as ciliary abnormality in this study.
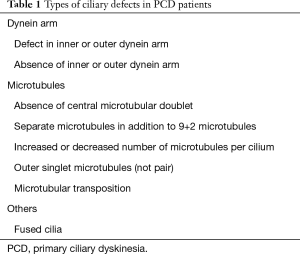
Full table
PCD was diagnosed when TEM showed ultrastructural abnormality consistent with PCD. When the ciliary structure was normal or not analyzable due to poor quality of the mucosal specimen or severe inflammation, we determined these cases as ‘non-diagnostic’.
Statistical analyses
Data are presented as median and range for continuous variables and as number (percentage) for categorical variables. Data were compared by Kruskal-Wallis test for continuous variables and by Pearson’s Chi-square test or Fischer’s exact test for categorical variables. All tests were two-sided, and P<0.05 was considered significant. Data were analyzed with IBM SPSS Statistics for Windows, version 24.0 (SPSS, Inc.).
Results
Baseline characteristics
Of 96 patients with biopsies for TEM, there were 59 in the NB group, 17 in the BB group, and 20 in the NBB group (Table 2). Patients in the NBB group were more likely to be older and current or ex-smokers than those in the NB and BB groups (P=0.002 and P=0.011, respectively). The presence of pneumonia history was lower in the NB group (69.5%) than in the BB (100%) and NBB groups (85.0%) (P=0.013). Cough/sputum were more common in the BB and NBB groups (P=0.018), and rhinorrhea was more common in the NB group (P<0.001). The presence of sinusitis was higher in the NB group than in the BB group and NBB group (P<0.001). No significant differences in sex, body mass index, history of otitis media, incidence of bronchiectasis, and inflammatory status measured with white blood cells and C-reactive protein were found among the three groups.
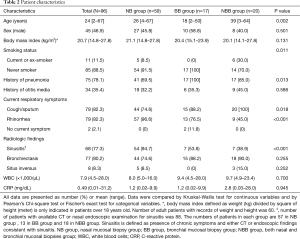
Full table
Diagnostic rate of PCD according to biopsy site
As shown in Figure 2, the diagnostic rate of PCD was 28.8% (17/59) in the NB group and 41.2% (7/17) in the BB group. In the NBB group, only 5 patients (25.0%) were diagnosed with PCD by nasal mucosal biopsy, and the diagnostic rate was similar to that of the NB group. However, 7 (35.0%) patients were additionally diagnosed with PCD by bronchial mucosal biopsy. Finally, 12 patients (60.0%) were diagnosed with PCD by biopsies at both sites (Figure 2).
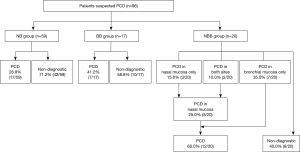
There was no significant difference in the diagnostic rate of PCD between the NB group and BB group (P=0.334) or between the BB group and NBB group (P=0.254). The diagnostic rate was significantly higher in the NBB group than in the NB group (P=0.012) (Figure 3A). The diagnostic rates in adult patients excluding pediatric patients (age over 18) also similar to those in all patients [NB group, 25% (9/36) vs. NBB group, 67% (12/18); P=0.003] (Figure 3B).

Regarding complications after bronchial mucosal biopsy, post-bronchoscopic fever was noticed in three patients (8.1%, 3/37), but there were no other significant complications after bronchoscopy such as bleeding or pneumothorax.
Presence of sinusitis and diagnostic rate of PCD by nasal mucosal biopsy
Among 79 patients with a nasal mucosal biopsy, 75 underwent OMU CT scan and/or nasal endoscopy (Figure 4A). A total of 61 patients (81%) had chronic sinusitis, and 14 patients had no symptoms of chronic sinusitis or CT/endoscopic findings consistent with sinusitis. As shown in Figure 4A, the presence of sinusitis was not associated with diagnostic rate of PCD [34% (21/61) vs. 50% (7/14); P=0.277].
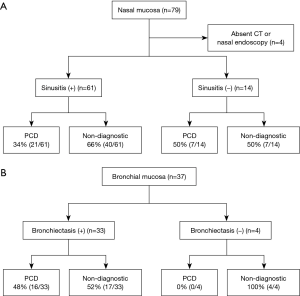
Presence of bronchiectasis and diagnostic rate of PCD by bronchial mucosal biopsy
All 37 patients who underwent bronchial mucosal biopsy had a chest CT scan. Among them, there was no evidence of bronchiectasis by chest CT in 4 patients, and none of their specimens showed ciliary structural abnormalities. As shown in Figure 4B, the diagnostic yield of PCD by bronchial mucosal biopsy was not significantly different between the patients with bronchiectasis and those without [48% (16/33) vs. 0% (0/4); P=0.118].
Discussion
To the best of our knowledge, this is the first study showing that bronchial mucosal biopsy provides additional diagnostic yield in patients with suspected PCD with negative TEM results by nasal mucosal biopsy. This retrospective study showed that diagnosis of PCD increased by up to 31% with combination of nasal mucosal biopsy and bronchial mucosal biopsy compared with nasal mucosal biopsy only. This study also showed that 35% of patients were additionally diagnosed with PCD by bronchial mucosal biopsy despite negative results in nasal mucosal biopsy, demonstrating the additional role of bronchial mucosal biopsy beyond nasal mucosal biopsy in patients with suspected PCD.
Despite the higher sensitivity of TEM in diagnosing PCD, the diagnostic rate of PCD by TEM with nasal mucosal biopsy alone was relatively low in patients with suspected PCD (9,11-15). In agreement with the results of previous studies, our study showed that the diagnostic rate of PCD by TEM with nasal mucosal biopsy was as low as 29%. Although the diagnostic rate of bronchial mucosal biopsy was 41%, this yield was not statistically higher than that of nasal mucosal biopsy, as shown in previous studies (8,10,21). These results suggest that TEM with a single biopsy site might not be sufficient to diagnose PCD. Although combination testing with HSVA or nNO can increase the accuracy of diagnosing PCD (11), those methods are not easily accessible for most clinics. Given significantly increased diagnostic yield for diagnosis of PCD with a combination of nasal mucosal biopsy with bronchial mucosal biopsy, the performance of both nasal and bronchial mucosal biopsies will be helpful to enhance diagnosis of PCD.
We further evaluated whether the presence of sinusitis predicts the diagnostic yield of PCD using nasal mucosal biopsy. We hypothesized that the presence of sinusitis is associated with increased diagnostic yield, but there was no significant difference in the diagnostic rate between patients with and without sinusitis. We also compared the diagnostic yield of PCD between patients with and without bronchiectasis. Although none of the specimens of patients without bronchiectasis showed abnormal ciliary structure, there was no significant difference in the diagnostic rate between patients with and without bronchiectasis on CT scan. Taken together, these results suggest that the presence or absence of sinusitis or bronchiectasis was not a significant factor to determine biopsy site for PCD in our study. Since there was a small number of patients, further prospective studies with larger numbers are needed to validate our findings.
Based on the results of our study, we suggest a novel diagnostic approach using TEM with a combination of nasal mucosal biopsy and bronchial mucosal biopsy (Figure 5). The diagnostic yield of bronchoscopic mucosal biopsy seemed to be higher than that of nasal mucosal biopsy, but there was no significant difference. In addition, the presence of sinusitis on imaging findings did not predict diagnosis of PCD. Given the less invasive technique for obtaining nasal mucosal biopsy (8), a sequential diagnostic approach using nasal mucosal biopsy as the first step, followed by bronchial mucosal biopsy would enhance diagnosis of PCD in clinics where only TEM is available for PCD diagnosis.

This study involves several limitations. First, given the small and retrospective design of this study in a single center, selection bias may have influenced the findings. Moreover, the patients who underwent NB, BB or NBB were not under similar clinical conditions in terms of clinical symptoms and radiologic findings. For example, NB group was more likely to have sinusitis compare with other two groups and BB and NBB group had more history of pneumonia than NB group. Thus, further study with randomized controlled design is required to confirm our study. Second, we did not use a combination of various methods in addition to TEM to diagnose PCD, such as nNO analysis, HSVA, genotyping, and immunofluorescence, as recommended in recent guidelines (5,6). However, most of these tests are not available even in tertiary hospitals, and TEM is a relatively established method for other medical or scientific purposes. Thus, TEM would be a feasible option to increase the rate of PCD diagnosis under this limited circumstance. In this way, our study provided valuable information that will enhance the diagnostic yield of TEM. Third, we could not provide the final diagnosis in patients with negative results in both nasal and bronchial mucosal biopsies. Thus, further studies that can suggest the diagnostic flow for these patients are needed. Lastly, ciliary ultrastructural abnormality in TEM could be secondary change due to inflammation, infection, irritation, or handling artifact (22). This secondary change, also called secondary ciliary defect, cannot be easily distinguished from PCD by TEM. Various typical features of primary ciliary defects and non-specific or secondary structural defects have been reported, but most of the primary defects can be identified in secondary changes (23). Quantitative analysis of TEM was suggested, but there are no established criteria to differentiate primary defects from secondary change (24). Secondary ciliary defect might be excluded by avoiding biopsy after recent inflammation or infection, but this does not guarantee observation of the primary defect of cilia (22). Cell culture can be used to reduce the risk of secondary ciliary defect (25) and it is strongly recommended in recent guidelines (5), but this test is also rarely available in clinical setting. Despite these limitations, our study showed a higher detection rate of ciliary ultrastructural abnormality and provides more clinical information for diagnosis of PCD.
In conclusion, the combination of nasal mucosal biopsy and bronchial mucosal biopsy yielded a higher diagnostic rate of PCD than the use of nasal mucosal biopsy only. Our data suggest that bronchial mucosal biopsy would have an additional role to increased PCD diagnosis in the clinical settings with only TEM for PCD diagnosis. However, further prospective studies with large numbers are required to confirm our results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Institutional Review Board of Samsung Medical Center (SMC 2018-04-029-001). Informed consent was waived for the use of patient medical data and patient information was anonymized and de-identified prior to analysis.
References
- Knowles MR, Daniels LA, Davis SD, et al. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 2013;188:913-22. [Crossref] [PubMed]
- Kuehni CE, Frischer T, Strippoli MP, et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in European children. Eur Respir J 2010;36:1248-58. [Crossref] [PubMed]
- Bush A, Chodhari R, Collins N, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child 2007;92:1136-40. [Crossref] [PubMed]
- Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J 1997;10:2376-9. [Crossref] [PubMed]
- Lucas JS, Barbato A, Collins SA, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 2017;49:1601090. [Crossref] [PubMed]
- Shapiro AJ, Davis SD, Polineni D, et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2018;197:e24-39. [Crossref] [PubMed]
- Afzelius BA. A human syndrome caused by immotile cilia. Science 1976;193:317-9. [Crossref] [PubMed]
- Adil EA, Kawai K, Dombrowski N, et al. Nasal versus tracheobronchial biopsies to diagnose primary ciliary dyskinesia: A meta-analysis. Laryngoscope 2017;127:6-13. [Crossref] [PubMed]
- Shoemark A, Dixon M, Corrin B, et al. Twenty-year review of quantitative transmission electron microscopy for the diagnosis of primary ciliary dyskinesia. J Clin Pathol 2012;65:267-71. [Crossref] [PubMed]
- Papon JF, Coste A, Roudot-Thoraval F, et al. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J 2010;35:1057-63. [Crossref] [PubMed]
- Haarman EG, Schmidts M. Accuracy of diagnostic testing in primary ciliary dyskinesia: are we there yet? Eur Respir J 2016;47:699-701. [Crossref] [PubMed]
- Munkholm M, Nielsen KG, Mortensen J. Clinical value of measurement of pulmonary radioaerosol mucociliary clearance in the work up of primary ciliary dyskinesia. EJNMMI Res 2015;5:118. [Crossref] [PubMed]
- Olin JT, Burns K, Carson JL, et al. Diagnostic yield of nasal scrape biopsies in primary ciliary dyskinesia: a multicenter experience. Pediatr Pulmonol 2011;46:483-8. [PubMed]
- Pifferi M, Caramella D, Cangiotti AM, et al. Nasal nitric oxide in atypical primary ciliary dyskinesia. Chest 2007;131:870-3. [Crossref] [PubMed]
- Stannard WA, Chilvers MA, Rutman AR, et al. Diagnostic testing of patients suspected of primary ciliary dyskinesia. Am J Respir Crit Care Med 2010;181:307-14. [Crossref] [PubMed]
- Dodd JD, Lavelle LP, Fabre A, et al. Imaging in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med 2015;36:194-206. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50:1-12. [Crossref] [PubMed]
- Escalier D, Jouannet P, David G. Abnormalities of the ciliary axonemal complex in children: An ultrastructural and cinetic study in a series of 34 cases. Biol Cell 1982;44:271-82.
- de Iongh RU, Rutland J. Ciliary defects in healthy subjects, bronchiectasis, and primary ciliary dyskinesia. Am J Respir Crit Care Med 1995;151:1559-67. [Crossref] [PubMed]
- Rossman CM, Lee RM, Forrest JB, et al. Nasal ciliary ultrastructure and function in patients with primary ciliary dyskinesia compared with that in normal subjects and in subjects with various respiratory diseases. Am Rev Respir Dis 1984;129:161-7. [PubMed]
- MacCormick J, Robb I, Kovesi T, et al. Optimal biopsy techniques in the diagnosis of primary ciliary dyskinesia. J Otolaryngol 2002;31:13-7. [Crossref] [PubMed]
- Dixon M, Shoemark A. Secondary defects detected by transmission electron microscopy in primary ciliary dyskinesia diagnostics. Ultrastruct Pathol 2017;41:390-8. [Crossref] [PubMed]
- Ibañez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet 2003;12:R27-35. [Crossref] [PubMed]
- Sirvanci S, Seda Uyan Z, Ercan F, et al. Quantitative analysis of ciliary ultrastructure in patients with primary ciliary dyskinesia. Acta Histochem 2008;110:34-41. [Crossref] [PubMed]
- Hirst RA, Jackson CL, Coles JL, et al. Culture of primary ciliary dyskinesia epithelial cells at air-liquid interface can alter ciliary phenotype but remains a robust and informative diagnostic aid. PLoS One 2014;9:e89675. [Crossref] [PubMed]


