
Does conversion from a minimally invasive to open procedure hurt the patient, the surgeon’s ego, or the healthcare system?
Dr. Fourdrain and colleagues have presented an interesting article that attempts to answer if intraoperative conversion to thoracotomy from a video-assisted thoracoscopic approach is harmful to patients. It is an important question, and few if any articles have attempted to answer it. This question has clinical value as well—if intraoperative conversion is harmful and leads to poorer patient outcomes, if it adds operative time and expense, or if the surgeon skill set or patient anatomy indicates that intraoperative conversion is more than likely—should the surgeon just start off open? How would this impact the learning curve that so many of us have started on? We have been taught to go as far as you can in a pulmonary resection with minimally invasive techniques [via video-assisted thoracoscopic surgery (VATS) or robotically] and then convert to thoracotomy only if absolutely necessary. We now know that minimally invasive platforms offer patients real advantages over thoracotomy, including decreased hospital length of stay, decreased operative time as surgeon skill improves, and lower rates of patient morbidity and mortality (1-3). We applaud the author’s question but fear the data set in this particular study does not allow us to adequately answer it.
Perhaps the most important theoretical consequence of conversion is the long-term effects a thoracotomy may have on long-term cancer survival or disease-free recurrence compared to a minimally invasive operation. This study is focuses only on short-term perioperative results. Second, the data presented in this article has some red flags. We do not mean to disparage the authors in any way, rather we congratulate them—but we have to mention some facts. Some of this data perhaps does not accurately reflect the current state of VATS even when taking into account a surgeon’s new learning curve, and thus we fear may not be translatable to the other thoracic surgical practices. The data points from the study that stand out include: (I) only 50% of patients were selected for VATS over thoracotomy (301 for VATS vs. 309 for thoracotomy); (II) only 7% of patients in the VATS and VATS conversion group combined had a T2b tumor; (III) there was an 18.1% conversion rate from VATS to thoracotomy; (IV) 109 of the initial 919 patients (11.9%) underwent pneumonectomy; and (V) the mortality rates for VATS with conversion were 1.8% at 30 days and 5.4% at 90 days.
These results are drastically different from our own practice even if we include our learning curve for the robotic approach: 98% of our patients are selected for robotics for elective lung cancer resection over thoracotomy, T2b tumors or larger represents 29% in our series using minimally invasive techniques, we have a 4% conversion rate from the robotic platform to open thoracotomy, a 1% pneumonectomy rate, a 0.3% 30-day and 0.6% 90-day mortality rate (4-6).
If we compare this study to other selected historic VATS series (Table 1), we see that open conversion rate, 30- and 90-day mortality rates are significantly higher in this study, even accounting for a learning curve inherent with a new surgical technique. Does this mean the comparison VATS group may be too different? If the patient population in this study is so drastically different from the majority of the published literature, can we safely answer the important question the authors have proposed? Perhaps a database study with larger numbers is warranted but only if we have reliable granular data on when operations where truly converted and if we can gather accurate 5-year follow-up data. Based on this article, all we can say for now is that when these surgeons examine short-term outcomes within their own practice, they have seen no disadvantageous effects when converting from VATS to open thoracotomy.
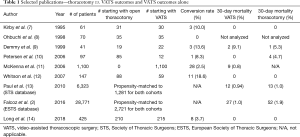
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Cerfolio discloses relationships with Bovie, Community Health Services, Covidien/Medtronic, C-SATS, Davol/Bard, Ethicon, Google/Verb, Intuitive Surgical, KCI/Acelity Company, Myriad Genetics, Pinnacle, TEGO Corporation and is the president of ROLO-7 consulting firm. Dr. Ferrari-Light has no conflicts of interest to declare.
References
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7.
- Smood B, Ghanim A, Wei B, et al. Technical and operational modifications required for evolving robotic programs performing anatomic pulmonary resection. J Robot Surg 2018;12:529-34. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Lobectomy--video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 1995;109:997-1001; discussion 1001-2. [Crossref] [PubMed]
- Ohbuchi T, Morikawa T, Takeuchi E, et al. Lobectomy: video-assisted thoracic surgery versus posterolateral thoracotomy. Jpn J Thorac Cardiovasc Surg 1998;46:519-22. [Crossref] [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214-8; discussion 219. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Long H, Tan Q, Luo Q, et al. Thoracoscopic Surgery Versus Thoracotomy for Lung Cancer: Short-Term Outcomes of a Randomized Trial. Ann Thorac Surg 2018;105:386-92. [Crossref] [PubMed]


