
Enhancing the study of enhanced recovery after thoracic surgery: methodology and population-based approaches for the future
Introduction
Enhanced recovery after surgery (ERAS) pathways are designed to improve postoperative recovery, reduce morbidity, and decrease length of stay (LOS). Pathways have elements in all phases of perioperative care including: preoperative (education, nutrition), intraoperative (minimally invasive surgery, effective regional anesthetic, minimization of drains), and postoperative (early ambulation, multimodal opioid-sparing analgesia, nutritional optimization) (1). These evidence-based ERAS pathways have been applied by a number of surgical subspecialties and are associated with decreased morbidity, LOS, and cost (2,3).
Recently, ERAS pathways have been successfully implemented in thoracic surgery for patients undergoing pulmonary resection. Studies have reported decreased LOS and decreased pulmonary and overall morbidity (4-9) (Table 1). However, the majority of these studies were retrospective reviews and the strength of conclusions limited due to small sample sizes and lack of randomization.
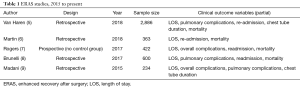
Full table
Despite these shortcomings, the data is compelling and as such the field of thoracic ERAS research has quickly grown as evidenced by a steady increase in publication volume. In this review we will briefly discuss the current state of thoracic enhanced recovery research, as well as discuss study design with a focus on methodology. Population based approaches and patient centered outcome measures will be discussed in the context of future thoracic ERAS studies.
ERAS studies prior to 2015
Improving patient outcomes with standardized treatment protocols, such as ‘fast track’ pathways, is not new to thoracic surgery
Thoracic ERAS studies prior to 2015 are summarized by a systematic review performed by Fiore et al. in 2016 (4). The authors concluded that the level of evidence was low and ERAS pathways did not significantly decrease overall complications, operative mortality, or hospital readmission. They emphasized that future studies evaluating ERAS in thoracic surgery should have improved trial design to provide conclusive evidence about its effectiveness. Nonetheless, these original studies provided the early framework for ERAS in thoracic surgery.
Modern ERAS studies
Since 2015, there have been several published studies evaluating the effectiveness of ERAS for thoracic surgery (5-9). The individual elements of ERAS pathways are well summarized in a recent review by Semenkovich et al. (11). Overall, the ERAS elements are heterogeneous, however as compared to the studies summarized by Fiore et al. (4) several key concepts are more uniform across series. ERAS development and implementation requires a multidisciplinary team including, but not limited to surgeons, anesthesiologists, nurses, and pharmacists. They incorporate elements that have been shown to improve outcomes in other surgical subspecialties such as colorectal surgery (1). These elements include: preoperative, intraoperative, and postoperative interventions. Postoperative pain management focuses on a multimodal, opioid sparing regimen, often using acetaminophen and nonsteroidal anti-inflammatory drugs.
Summary of outcomes
Primary outcome variables in the current studies included morbidity, readmission, LOS, cost, and mortality, among others. Van Haren et al. reviewed 2,866 lung resections; for patients undergoing thoracotomy, ERAS lead to to a decrease in LOS, pneumonia, atrial fibrillation, and need for discharge on home oxygen. No difference was seen in hospital readmission or mortality rates (5). Madani et al. evaluated 107 patients undergoing thoracotomy and lobectomy and found similar outcomes with respect to LOS and perioperative morbidity, mortality. and hospital readmission (9). Both overall cost and opioid use (morphine) were reduced in the study by Martin et al, including both VATS and thoracotomy patients (6). The study by Brunelli et al. did not identify any outcome benefit for VATS patients, however, numerous elements of the ERAS pathway were already effectively standard of care within the program (8). A single study assessed the effect of protocol compliance on outcomes, concluding that compliance with ERAS pathway was independently associated with decreased mortality (7 ). To summarize, implementation of ERAS appears both safe and feasible in thoracic surgery with no adverse increase in complications, readmission or mortality rates. These results suggest that when implemented, ERAS protocols are associated with improvements in perioperative clinical outcomes following thoracic surgery.
Research methodology
As reviewed, to date the primary methodology for ERAS focused thoracic surgical outcomes research has been single center retrospective review utilizing historical controls (5-9). Aside from a single study where post-operative QOL was assessed via a SF-36 questionnaire, and a single randomized controlled trial (12,13) (“fast track” post-op clinical pathway as opposed to multidisciplinary ERAS protocol) studies have used traditional institutional datasets, with relatively straightforward statistical methodology. Recognizing that ERAS is a new care model for general thoracic surgery it is not surprising that the data available is limited. By necessity, a retrospective study design is appropriate, and simply by the nature of ERAS being often broadly applied to a variety of different case types (open/VATS, wedge/lobe, primary/metastatic, etc…) even with single center studies, the sample sizes can be quite large allowing for identification of small, but significant, clinical outcome differences. As mentioned above, the MD Anderson study, for example, included 2,866 patients, 1,271 in the transitional or ERAS group (5). Although a large sample alone is not sufficient to overcome the potential bias of a retrospective design, it does assist with analysis of the large number of elements within the protocols themselves. As Rogers and colleagues demonstrated, protocol compliance appeared to be associated with a decrease in perioperative morbidity and discharge delays; compliance in this series involved 15 discrete ERAS protocol elements (7). Multivariable regression analysis offers insight into the contribution of individual ERAS elements to clinical outcomes; when applied in this case, only 2 of the ERAS elements were individually predictive of a prolonged LOS, while none were predictive of 30-day morbidity. Perhaps more importantly, the compliance score for all ERAS elements was predictive of both outcomes. Regarding analysis of ERAS protocol elements, the whole may be greater than the sum of the parts, and this should be accounted for when determining the method of data analysis for a given study. These early results function as a reasonable jumping off point to help further define/refine the approach to ERAS research.
Currently there is no “proven” thoracic ERAS protocol and so the elements although relatively uniform, vary slightly between institutions. The previously discussed systematic review of enhanced recovery pathways (ERP) in elective lung resection identified 15 discrete protocol elements, with each study including between 4 and 10 (median 6.5), suggesting that not all ERAS protocols, and therefore studies, are created equal (4). Most studies have used the same outcome metrics, (LOS, 30-day mortality, chest tube duration, re-admission, cardiopulmonary complications), cost of intervention/cost-saving has also been reported, but less frequently. Patient reported outcomes (PRO) and patient-centered outcome measures (PCOM) have been infrequently reported although, in one study post-operative quality of life (QOL) as determined using the Short-Form 36 survey (SF-36) supported use of ERP. With variations in the care patterns across institutions as well as variation in any individual ERAS protocol, multi-institutional studies have yet to be performed.
Current level of evidence
Based on the design and methodology of the most recent studies, the level of evidence to support the use of ERAS protocols remains limited. That said, the studies do present compelling data despite the shortcomings. Certainly some of the more significant critiques across all studies are the use of historical controls and variability of protocol elements. The inclusion of specific ERAS elements in one study may reflect the current standard of care at another institution, thus the contribution of ERAS to typical clinical outcome metrics may be masked. Study results support ERAS protocols as safe and impactful when applied to patients undergoing traditional open approaches for surgical diseases of the chest, though when applied to minimally invasive procedures, the outcomes are less impressive (8). The use of historical controls may have also contributed to a significant bias in favor of the ERAS intervention groups when both VATS and open procedures were included, as the utilization of VATS has continued to increase with time (14). Those studies identifying an improvement in LOS did so primarily when analyzing the thoracotomy or open cohorts separately. Programs performing high-volume minimally-invasive lung resections may see no significant alteration in clinical outcome metrics. Although with minimally invasive procedures (VATS/robotic) we may be approaching the asymptote of the curves for LOS, perioperative morbidity, 30-day mortality, etc. that does not imply that no further improvements can be had; redefined study endpoints and alternate methodology are needed. Evidence from other surgical specialties suggest that even absent an ERAS protocol, simply limiting practice variation alone via guideline concordant care can lead to improved surgical outcomes (15), suggesting the contribution of ERAS to outcomes is more complex than current studies are able to define.
In that context, compliance with a newly implemented ERAS protocol may be a more relevant question than is represented in the literature; as Martin et al describe, the implementation of a new ERAS protocol is an iterative process with corrections occurring in “real-time” as issues arose (6). The dynamic nature of process implementation represents an additional source of time-dependent bias when using hard start/stop points for sample selection. In an attempt to mitigate this bias Van Haren et al. utilized a “transitional” cohort, in addition to the pre- and post-ERAS groups (5). Interestingly, the transitional cohort with respect to pulmonary complications effectively mirrored the pre-ERAS group, suggesting again that compliance was necessary for an overall effect to be identified. By focusing on limiting care variance, the effects of an ERAS program may be less related to the individual protocol elements, and more a function of consistent practice on a programmatic/system level. Taken in aggregate, the results are compelling although perhaps not convincing, with the level of evidence in support of ERAS being limited. More importantly, the current work enables the researcher to identify knowledge gaps and directions for future research.
Future directions
Further work is required to validate and build upon the early results of ERAS protocol implementation for thoracic surgery. The early data shows promise, but has also identified a need for an alternate approach to traditional outcomes research. Is the next iteration of study for ERAS a randomized controlled trial? Although certainly the gold standard, and providing the highest level of evidence, research into ERAS pathways may not necessarily lend itself well to a RCT. The complexity of interventions, dependent on factors from patient to system level, make a RCT a challenging proposition. Further, refinement of the protocols themselves as well as validated trial endpoints will be necessary for an effective study design. Interrupted time series studies remain a valuable tool for ERAS research on the system level, and when well designed, outcomes may mirror those of RCT’s (16).
To date there have been no population-based studies focusing on thoracic ERAS, although this may be an optimal mechanism to identify the overall “value” of an ERAS protocol. Three approaches to ERAS based work appear highest yield: (I) identification of impactful study end-points, (II) optimal protocol element inclusion (III) protocol compliance and implementation.
Impactful study endpoints
A population based approach may offer the best opportunity to identify the effect of an ERAS pathways when implemented in a system-wide fashion. As an example, with the dramatic increase in prescription opioid related deaths, there is an increased focus on the healthcare industries contribution to opioid abuse following surgical procedures. Patients undergoing surgery either open or VATS for non-small cell lung cancer appear especially at risk, with up to 15% of patients becoming long-term opiate users (17). Extrapolating from the number of patients undergoing curative intent surgery for non-small cell lung cancer, this equates to thousands of new, chronic opioid users yearly. Fortunately, a cornerstone of ERAS protocols is an opioid sparing pain regimen. Inpatient opioid equivalent use, opioids required on discharge, number of prescription refills, and long-term opioid use all represent measureable and clinically meaningful endpoints where ERAS protocols will likely have a significant impact. This can be coupled to the indirect impact ERAS may have on disparities in care provided to vulnerable or minority populations. Taking the lead from our colorectal surgical colleagues, following implementation of an ERAS protocol, in addition to an overall reduction in LOS, racial differences in post-operative outcomes, specifically LOS, were effectively eliminated (18). Similarly, multiple studies have demonstrated disparities in outcomes for patients with NSCLC related to race, ethnicity and socioeconomic status (SES) (19-21); this represents a valuable and potentially broadly impactful end-point for thoracic ERAS.
Inclusion of PRO as well as PCOM are essential components to quality comparative effectiveness research. Regarding thoracic surgery, The American College of Chest Physicians guidelines recommend a “validated health-related QOL instrument be used at baseline clinic visits and during follow-up” for patients who have undergone curative intent treatment for non-small cell lung cancer (22). With the ideal goal of ERAS to be health-care system level implementation, collection and analysis of PRO/PCOM contributes greatly to the strength of outcomes research. There are number of validated instruments available for collection of PRO, and a recent pilot study utilizing item banks from the NIH Patient Reported Outcome Measurement Information System (PROMIS) concluded that this data could be (and likely will be) collected and integrated into large national databases such as the STS general thoracic surgery database (23). With broad implementation of ERAS protocols and collection of PRO/PCOM coupled to traditional outcome measures in a large national dataset, the opportunities for meaning outcomes research are vast. Arguing for broad implementation of ERAS protocols requires that in addition to being safe and clinically effective, they be cost-effective. Mirroring studies of colorectal procedures; modeling of direct variable cost based on projected reductions in hospital LOS adjusted by institutional case-load and complexity represents an additional end-point for focused thoracic ERAS research (24). A single Canadian study uniquely assessed institutional, health system, and societal cost in addition to clinical outcome metrics. The ERP was associated with a significant decrease in societal cost [$−4,396 (−8,674 to 681)], for patients with major complications that cost savings was even greater [−10,946 (−17,145 to −4,697)] (25). Earlier return to work, fewer days requiring home caregivers, either family or hired, contribute to the downstream value of ERAS protocols, with the effects being especially significant in vulnerable populations where a short-term loss of income can be potentially catastrophic.
Optimal protocol elements
As previously noted, there is no consensus as to what constitutes an optimal thoracic surgery ERAS protocol. Early iterations of thoracic ERAS have attempted to build on the success of other surgical specialties (1-3). Although this has allowed for a streamlined effort to create and implement thoracic ERAS by mirroring prior successes, a rigorous review of the interventions themselves in context is still needed. Coupled with identification of appropriate outcomes measures, the elements of any ERAS protocol should be measured by their ability to affect the selected outcomes. As an example, with respect to element inclusion; for institutions/individuals with early ERAS experience, the Delphi method can be used to identify essential components of an ERAS protocol providing consistency across programs and thus allow for multi-institutional studies to take place.
Compliance and implementation
Protocol compliance appears to be correlated with the clinical effectiveness of ERAS protocols (7,26,27). Recognizing this, the researcher can approach the question from several directions; to what degree does compliance matter in an “optimal” thoracic ERASE protocol? Is there a single element whereby the entire protocol depends? Regression tree analyses of protocol elements can be used to identify “cut-points” necessary for protocol effectiveness. How to achieve this necessary degree of protocol adherence is another open opportunity for research. Tangential to this is the field of implementation science, which in the context of healthcare aims to study and promote the systematic adoption of best-evidence into clinical practice. Research looking to identify barriers to implementation (provider, patient, system, etc…) is necessary, as conclusions drawn based on studies of ERAS where compliance is suboptimal are of limited value. A study of perceived barriers and enablers to ERAS implementation in a multisite colorectal program identified protocol elements which were already considered standard of care, where effectively no barriers existed, while others elements were identified as problematic with resistance to implementation from all involved disciplines (surgeon, anesthesiology, nursing) (28). Martin and colleagues nicely outlined the implementation pathway, barriers, and the dynamic process improvements upon the first-year of implementing a thoracic ERAS pathway. Alternatively, the question can be approached from a population-based aspect, evaluating the association of race, SES, gender, or geography (urban/rurual), amongst others, with adherence to ERAS program elements. Studies of colorectal surgery patients suggested that compliance with pre-operative fasting was reduced in African-American patients as compared to white patients (32% vs. 47%) (18), while individuals of a high SES as compared to low SES were more likely to be compliant with the ERAS protocol elements (32% vs. 17%) (29). In this context studies focused on identifying interventions to improve protocol adherence among at-risk populations is likely to be high-yield with respect outcome improvements secondary to ERAS. Once again, coupling with PRO and protocol element refinement, studies can focus on how to successfully integrate new best-evidence into a functional ERAS protocols.
Conclusions
High quality data supporting ERAS for patients undergoing thoracic surgery is limited, but early studies are compelling and support further investigation into ERAS protocols and implementation. The multimodal, multidisciplinary, and longitudinal nature of ERAS protocols affords itself to broad spectrum of outcomes-based research from traditional clinical outcomes, patient centered outcomes, to systems implementation. The shifting landscape of healthcare delivery, particularly with respect to oncology, well positions ERAS focused researchers for novel and impactful future studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Varadhan KK, Neal KR, Dejong CH, et al. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010;29:434-40. [Crossref] [PubMed]
- Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38:1531-41. [Crossref] [PubMed]
- Fiore JF Jr, Bejjani J, Conrad K, et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg 2016;151:708-15.e6. [Crossref] [PubMed]
- Van Haren RM, Mehran RJ, Mena GE, et al. Enhanced Recovery Decreases Pulmonary and Cardiac Complications Following Thoracotomy for Lung Cancer. Ann Thorac Surg 2018;106:272-9. [Crossref] [PubMed]
- Martin LW, Sarosiek BM, Harrison MA, et al. Implementing a thoracic enhanced recovery program: Lessons learned in the first year. Ann Thorac Surg 2018;105:1597-604. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [PubMed]
- Madani A, Fiore JF Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908; discussion 908-10. [Crossref] [PubMed]
- Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [Crossref] [PubMed]
- Semenkovich TR, Hudson JL, Subramanian M, et al. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg 2018;30:342-9. [Crossref] [PubMed]
- Muehling BM, Halter GL, Schelzig HS, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [Crossref] [PubMed]
- Numan RC, Klomp HM, Li W, et al. A clinical audit in a multidisciplinary care path for thoracic surgery: an instrument for continuous quality improvement. Lung Cancer 2012;78:270-5. [Crossref] [PubMed]
- Detterbeck F, Molins L. Video-assisted thoracic surgery and open chest surgery in lung cancer treatment: present and future. J Vis Surg 2016;2:173. [Crossref] [PubMed]
- Reames BN, Shubeck SP, Birkmeyer JD. Strategies for Reducing Regional Variation in the Use of Surgery A Systematic Review. Ann Surg 2014;259:616-27. [Crossref] [PubMed]
- Fretheim A, Zhang F, Ross-Degnan D, et al. A reanalysis of cluster randomized trials showed interrupted time-series studies were valuable in health system evaluation. J Clin Epidemiol 2015;68:324-33. [Crossref] [PubMed]
- Tuminello S, Schwartz RM, Liu B, et al. Opioid Use After Open Resection or Video-Assisted Thoracoscopic Surgery for Early-Stage Lung Cancer. JAMA Oncol 2018;4:1611-3. [Crossref] [PubMed]
- Wahl TS, Goss LE, Moris MS, et al. Enhanced Recovery After Surgery (ERAS) Eliminated Racial Disparities in Postoperaive Length of Stay After Colorectal Surgery. Ann Surg 2018;268:1026-35. [Crossref] [PubMed]
- Tannenbaum SL, Koru-Sengul T, Zhao W, et al. Survival disparities in non-small cell lung cancer by race, ethnicity and socioeconomic status. Cancer J 2014;20:237-45. [Crossref] [PubMed]
- Mehta AJ, Stock S, Gray SW, et al. Factors contributing to disparities in mortality among patients with non-small-cell lung cancer. Cancer Med 2018;7:5832-42. [Crossref] [PubMed]
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341:1198-205. [Crossref] [PubMed]
- Colt HG, Murgu SD, Korst RJ, et al. Follow-up and Surveillance of the Patient with Lung Cancer After Curative-Intent Therapy: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e437S-54S.
- Khullar OV, Rajaei MH, Force SD, et al. Pilot Study to Integrate Patient Reported Outcomes After Lung Cancer Operations Into The Society of Thoracic Surgeons Database. Ann Thorac Surg 2017;104:245-53. [Crossref] [PubMed]
- Stone AB, Grant MC, Roda CP, et al. Implementation Cost of an Enhanced Recovery After Surgery Program in the United States: A Financial Model and Sensitivity Analysis Based on Experiences at a Quaternary Academic Medical Center. J Am Coll Surg 2016;222:219-25. [Crossref] [PubMed]
- Paci P, Madani A, Lee L, et al. Economic Impact of an Enhanced Recovery Pathway for Lung Resection. Ann Thorac Surg 2017;104:950-7. [Crossref] [PubMed]
- Pędziwiatr M, Kisialeuski M, Wierdak M, et al. Early implementation of Enhanced Recovery After Surgery (ERAS®) protocol - Compliance improves outcomes: A prospective cohort study. Int J Surg 2015;21:75-81. [Crossref] [PubMed]
- ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg 2015;261:1153-9. [Crossref] [PubMed]
- Pearsall EA, Meghji Z, Pitzul KB, et al. A Aulitiative Study to Understand the Barriers and Enablers in Implementing an Enhanced Recovery After Surgery Program. Ann Surg 2015;261:92-6. [Crossref] [PubMed]
- Leeds IL, Alimi Y, Hobson DR, et al. Racial and Socioeconomic Differences Manifest in Process Measure Adherence for Enhanced Recovery After Surgery Pathway. Dis Colon Rectum 2017;60:1092-101. [Crossref] [PubMed]


