Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for esophageal squamous cell cancer patients presenting with oligometastases
Introduction
Long-term survival of patients with stage IV esophageal cancer is rare, with less than 5% surviving 5 years (1). Only palliative chemotherapy and supportive measures are recommended by current National Comprehensive Cancer Network guidelines (2).
A recent observational cohort study (3) assessed the survival outcomes in metastatic esophageal cancer patients (3/4 of whom had adenocarcinomas) who received radiotherapy directed at the primary tumor, and the results showed that patients undergoing chemotherapy plus definitive-dose radiotherapy had an improved survival compared to those receiving chemotherapy alone (median overall survival 11.3 vs. 8.3 months; P<0.001). In another study, Saddoughi and colleagues from the Mayo Clinic analyzed the outcomes of patients with stage IV esophageal cancer patients with good performance status who were treated with surgery (4). There were few long-term survivors with this approach, leading the authors to recommend against surgery and to suggest instead—in the absence of evidence—that definitive chemoradiation might be a preferred strategy for stage IV esophageal cancer patients.
Therefore, data to support a benefit for adding radiation to all sites of disease in addition to chemotherapy for oligometastatic esophageal cancer patients are entirely lacking. We performed this retrospective analysis to evaluate the response rates and survival outcomes after concurrent chemoradiotherapy (CCRT) compared to chemotherapy alone for stage IV esophageal cancer patients with ≤3 metastases.
Methods
Patient selection
The subjects were recruited from the database of patients who were treated at Renmin Hospital of Wuhan University and Zhengzhou University Affiliated Cancer Hospital from January 2012 to December 2015. This study was approved by the Medical Ethics Review Committee of Renmin Hospital of Wuhan University, and the Number was 2017K-C023, and informed consent for participation was exempted by the board due to its retrospective nature.
At baseline, evaluation of Eastern Cooperative Oncology Group (ECOG) performance status (PS), basic blood work and enhanced cervical/thoracic/abdominal CT, barium swallow radiography were carried out. Patients were included if they met the following criteria: (I) diagnosis of pathologically confirmed esophageal squamous cell carcinoma or adenocarcinoma; (II) stage IV disease according to the AJCC 7th edition [2009] TNM (tumor, node, metastasis) classification (5); (III) ≤3 measurable metastases; (IV) aged 18–75 years; (V) ECOG-PS ≤2; (VI) chemotherapy regimen consisted of cisplatin/paclitaxel.
Patient cohorts were divided into two groups: patients who underwent CCRT to the primary tumor and all metastases as the initial treatment were included in the CCRT group, and patients who underwent chemotherapy (CT) alone during the entire treatment course were included in the CT group. The selection of treatment strategy was decided by treating physician, according to the risk/benefit profile based on the most updated knowledge at the time of therapy and the physician’s judgement.
Treatment regimen
CCRT cohort
Radiation therapy was delivered via high-energy (≥6 MV) linear accelerators. The clinical target volume extended 2 cm superior and inferior to the primary tumor, with a lateral margin of 1 cm, and a total radiation dose of 50 Gy/25 fractions was delivered over 5 weeks (5 days per week) using intensity-modulated radiotherapy (IMRT). The radiation field for metastases was created by adding 1 cm margin around metastatic lesions, and a radiation dose of 45 Gy/15 fractions was planned (5 days per week) using IMRT.
For the cisplatin/paclitaxel regimen, an intravenous 3-hour infusion of paclitaxel 135 mg/m2 on Day 1 and cisplatin 25 mg/m2 on Days 1–3 were given. Dexamethasone 20 mg, diphenhydramine 50 mg and ranitidine 50 mg were given intravenously 30 minutes prior to paclitaxel infusion. This regimen was repeated every four weeks for two cycles during radiation. The same regimen was then continued until disease progression, unacceptable toxicity or patient’s refusal to continue.
CT alone cohort
Among the patients in the CT alone group, the same chemotherapy regimen was administered every 4 weeks until disease progression, unacceptable toxicity or patient’s refusal to continue.
Response and toxicity evaluation
Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) system (version 1.1) (6). Because the purpose of the study was to investigate a non-surgical treatment for esophageal cancer patients, tumor responses were evaluated by thoracic and abdominal CT scan, and pathologic determination of primary tumor response was not required. However, endoscopic examination was performed if there were new symptoms suggestive of local failure. Response assessments were performed every 4–6 weeks after the initiation of treatment. Dysphagia score as described previously (7) was utilized to assess the grade of dysphagia, and a decrease of ≥1 point in dysphagia score was regarded as improvement of dysphagia. Dysphagia score was noted every 2 weeks after the initiation of treatment. Toxicities were assessed according to the Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE) (8).
Statistics
Statistical analysis was performed using SPSS for Windows, version 21.0. Descriptive statistics for discrete variables were noted as number and percentage.
The differences of patient and tumor characteristics, tumor response, dysphagia improvement, and treatment toxicity between the two groups were tested by Chi-square method. Progression was defined as an increase in either the size of primary tumor or metastatic lesions, or an increase in the number of metastatic lesions according to RECIST.
Progression-free survival (PFS) was assessed from the date of chemotherapy to the detection of disease progression or death from any cause. Overall survival (OS) was calculated from the date of chemotherapy until death or the last follow-up day in survivors. The survival curves were constructed using the Kaplan-Meier method, and differences between the curves were estimated by the log-rank test. Prognostic factors for PFS and OS were evaluated with univariate and multivariate Cox proportional hazards regressions, and all factors were included in multivariate regressions, regardless of their univariate significance level. All tests were two-sided, and P value less than 0.05 was considered significant.
Results
Patient characteristics
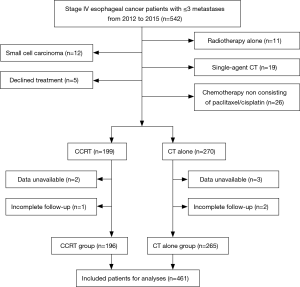
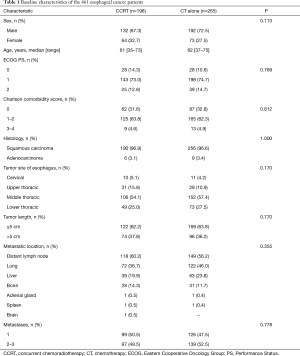
From January 2012 to December 2015, 542 consecutive stage IV esophageal cancer patients with ≤3 metastases were screened for the study. Among these patients, 12 had histologically proven small cell carcinoma, 5 declined any treatment, 11 received radiotherapy alone, 19 received single-agent chemotherapy, 26 did not receive chemotherapy that contained the paclitaxel/cisplatin regimen, data were unavailable for 5 patients, and 3 had incomplete follow-up; these 81 patients were excluded (Figure 1). As a result, a total of 461 patients were included in the final analysis. The majority of patients (70.3%) was male, and aged >60 years (58.4%), 96.7% had tumors of squamous cell histology, 56.0% had tumors located in the middle thoracic esophagus. One hundred ninety-six patients underwent CCRT, and 265 received CT alone. The characteristics of the 461 patients are shown in Table 1, and the baseline characteristics showed a similar distribution in the two groups.
Full table
Treatment compliance
In the CCRT group, 181 patients (92.3%) completed the planned RT. Radiation doses were reduced (40–46 Gy/20–23 fractions to the primary tumor, 30–45 Gy/12–18 fractions to metastases) in 15 patients and chemotherapy dose was reduced in 19 patients because of infection, esophagitis or hematologic toxicity. Twenty-six patients (13.3%) had their treatment interrupted <5 days because of toxicity.
Among the patients in the CT alone group, 26 (9.8%) received 2 chemotherapy cycles, 87 (32.8%) received 4 chemotherapy cycles, and 152 (57.4%) received ≥5 cycles of chemotherapy, and the median number of chemotherapy cycles was 6 (range, 2–12 cycles).
Evaluation of tumor response and dysphagia
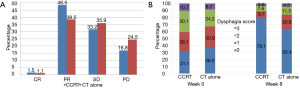
The overall response of primary tumor and metastatic lesions are shown in Figure 2A. At week 8, 3 patients (1.5%) achieved a complete response (CR), 95 (48.5%) achieved a partial response (PR), and 65 (33.2%) had stable disease (SD) in the CCRT group. CR, PR, and SD were observed in 3 (1.1%), 102 (38.5%), and 95 patients (35.9%), respectively, in the CT alone group. A statistically significant difference was demonstrated in the disease control rate (DCR; CR+PR+SD) between the two groups (83.2% vs. 75.5%, P=0.045). The analysis of dysphagia is shown in Figure 2B. At week 8, 78.5% of patients (106/135) in the CCRT group had improvement in their overall dysphagia score versus 61.5% (104/169) in the CT alone group, P=0.014.
Survival analysis
The median follow-up was 23.0 months (range, 3.5–50 months) for living patients and 11.5 months (range, 2.0–50 months) overall. For the total of 461 patients, the 0.5-, 1-, and 2-year PFS and OS rates were 62.8%, 25.1%, 2.2%, and 95.4%, 67.3%, 21.5%, respectively. The median PFS and OS were 7.8 months [95% confidence interval (CI), 7.1–8.5) and 15.9 months (95% CI, 14.8–17.0 months), respectively.
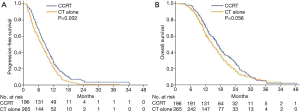
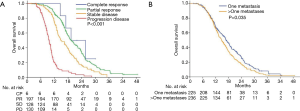
During the study period, 88.3% of patients in the CCRT group and 92.8% in the CT alone group experienced disease progression, which corresponded to a median PFS of 8.7 months (95% CI, 7.6–9.8 months) and 7.3 months (95% CI, 6.2–8.4 months), respectively. The 6-month, 1-year, and 2-year PFS rates were 68.1%, 27.6%, and 4.7%, respectively, in the CCRT group, and 58.0%, 21.9%, and 0.9%, respectively, in the CT alone group [hazard ratio (HR), 1.36; 95% CI, 1.12–1.66; P=0.002; Figure 3A). The median OS was 16.8 months (95% CI, 15.5–18.1 months) in the CCRT group and 14.8 months (95% CI, 13.2–16.4 months) in the CT alone group. The 1-, 2-, and 3-year survival rates were 72.8%, 27.2%, and 5.2%, respectively, in the CCRT group, and 63.5%, 17.5%, and 4.5%, respectively, in the CT alone group (HR, 1.23; 95% CI, 1.01–1.52; P=0.056; Figure 3B).
Evaluation of survival and prognostic factors
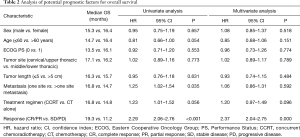
Table 2 shows the results of the analysis of prognostic factors for OS. Tumor response was significantly correlated to improved OS. As shown in Figure 4A, the median OS was 19.3 months in patients who achieved CR/PR. In contrast, median OS of 14.9 and 9.6 months was observed for patients who had SD and PD, respectively (HR, 2.29; 95% CI, 2.06–2.76; P<0.001). Patients with one metastasis had significantly longer OS compared with those had more than one metastasis, with median OS of 16.8 vs. 14.7 months, respectively (HR, 1.25; 95% CI, 1.02–1.54; P=0.035; Figure 4B).
Full table
The impact of patient, tumor, and treatment characteristics on OS were investigated by multivariate Cox regression analysis, and only tumor response (CR/PR vs. SD/PD; P<0.001) was significantly related to OS. Sex, age, PS, Charlson comorbidity score, tumor location, tumor length, number of metastases, and treatment regimen were not significantly related to OS.
Toxicity
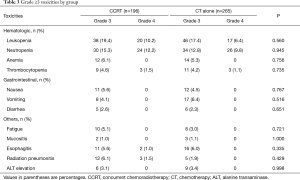
Grade 3 and 4 toxicity profiles are summarized in Table 3, and toxicities were generally mild. Leukopenia and/or neutropenia were the most common hematological toxicity, with 24.5% of the patients experiencing grade 3 or 4 toxicity. The incidences of leukopenia and neutropenia were slightly higher in CCRT group than that in CT alone group, but the difference did not reach statistical significance (P>0.05).
Full table
Non-hematological toxicities were comparable between the two groups. Death within 30 days of completion of CCRT was observed in 1 patient (0.5%).
Discussion
Metastatic esophageal cancer is generally considered a non-surgical disease because of the non-curative nature of this morbid procedure. However, clinicians have considered chemotherapy with definitive-intent radiotherapy to all sites for patients with good performance status who have limited sites of metastasis (9,10). Our study suggests that chemoradiation may be associated with a higher disease control and dysphagia improvement rate, as well as modest improvement in PFS and a trend toward better OS for esophageal squamous cell cancer patients with <3 metastases, as compared with chemotherapy alone.
Often, primary tumor progression in esophageal cancer can lead to dysphagia and subsequent malnutrition, chronic bleeding, and invasion of adjacent vital organs. Primary tumor local control may therefore play a positive role in the symptom relief and survival outcomes of patients with newly diagnosed metastatic esophageal cancer. The randomized TROG 03.01 phase III trial (11) compared radiotherapy to chemoradiotherapy to the primary tumor in 220 patients with metastatic esophageal cancer, and a palliative radiation dose range from 30 to 35 Gy was administered to the primary tumor. The patients receiving radiotherapy alone showed a dysphagia response of 67.9% vs. 73.9% in those who received chemoradiotherapy (P=0.13), and the median OS was 6.7 and 6.9 months respectively (P=0.88). Thus, the incorporation of palliative radiotherapy into the systemic therapy for metastatic esophageal cancer appears to convey minimal benefit. However, survival in this study cannot be clearly interpreted since patients in the chemoradiotherapy group only received chemotherapy during radiation, while those in the radiotherapy group did not receive any protocol-specified chemotherapy; the remarkably low OS likely reflects this non-standard aspect of the study.
Recently, Guttmann and colleagues (3) evaluated the impact of radiation dose on survival in metastatic esophageal cancer, and found that chemotherapy plus definitive-dose radiotherapy (≥50.4 Gy) was associated with improved overall survival OS compared to chemotherapy alone (11.3 vs. 8.3 months; P<0.001). In contrast, patients who received chemotherapy plus palliative dose radiotherapy had inferior outcomes with a median OS of 7.5 months. These data suggest that definitive rather than palliative doses of radiation are more likely to improve survival when combined with chemotherapy in metastatic esophageal cancer and may explain the lack of benefit for adding radiation in the TROG 03.01 study above.
Dysphagia is a distressing symptom and complex management problem in advanced esophageal cancer, and there is no consensus on the ideal treatment approach. Radiotherapy takes at least 2 weeks to produce alleviation of dysphagia, but its effect is more durable than that provided by other palliative approaches since radiotherapy treats the underlying primary tumor, not just the symptom. In an analysis (12) from Mansoura University Hospital, metastatic esophageal cancer patients with dysphagia were treated with radiotherapy (40 Gy/22 fractions) and concomitant chemotherapy (5-fluorouracil/cisplatin), 72% of patients showed dysphagia relief and the median duration of dysphagia improvement reached 5 months. A recent study (13) from Japan assessed the role of radiotherapy in the local management of stage IVB esophageal cancer. A rate of improvement in dysphagia score of 73% was noted, and the median nutritional support-free survival was 5 months. Ikeda et al. (14) reported 75% of patients with dysphagia improvement after CCRT, and the median nutrition-support-free survival reached 10 months. Dysphagia improvement seems to be about 70–75% for all these studies. In our study, the dysphagia improvement rates were 78.5% of patients after CCRT vs. 61.5% in patients who received CT alone. Therefore, CCRT may more effectively induce primary tumor regression, which results in greater improvement of dysphagia.
Dysphagia score and appetite loss can affect the quality of life and survival in patients with esophageal cancer (15), radiotherapy can effectively alleviate dysphagia and prolong nutrition-support-free survival. There is benefit for chemoradiation in patients with metastatic esophageal cancer, but the timing of the radiation remains undefined. For example, Hingorani and colleagues (16) added palliative radiotherapy in patients with metastatic esophageal and gastric cancer who had well-controlled metastatic disease after initial chemotherapy. The median PFS and OS for patients treated with palliative radiotherapy were 14 and 23.3 months, which were significantly higher than the 9.5 and 14 months, respectively, in patients treated with palliative chemotherapy alone. This study would suggest that an alternative strategy is to treat such patients initially with chemotherapy and to then add radiation to those patients who experience persistent dysphagia and who do not experience progression after systemic therapy.
In our study, patients who underwent CCRT achieved a median PFS of 8.7 months and a median OS of 16.8 months, in contrast, those who received CT alone had inferior outcomes with a median PFS of 7.3 months and a median OS of 14.8 months. Moreover, the patients who received CCRT tolerated the treatment regimen well, toxicities were generally mild, with no grade 5 radiation-induced pneumonitis or esophagitis observed, indicating that radiotherapy for primary tumor and metastatic lesions is clinically feasible. The impacts of Charlson comorbidity score on the treatment outcomes were also analysed, the median age was 62 years old, and only <5% of the enrolled patients had a Charlson comorbidity score of 3–4, the correlation between Charlson comorbidity score and the prognosis of patients was not found in our study.
The primary limitations of this study include its retrospective nature and the lack or randomization between the two treatment strategies; in fact, patients were selected based on their performance status/physician preference, which could bias the results in favor of the chemoradiation arm. The patients were also treated at two institutions and their favorable OS (15.9 vs. the 10–12 months seen in larger cohorts) suggests a highly selected group, likely largely because of their relatively small-volume metastatic disease. In addition to the fact that virtually all the patients had squamous cell carcinoma, these results should not be generalized to other patients with more extensive metastases or to those with adenocarcinomas.
The optimal treatment for esophageal cancer patients with limited metastatic disease has been controversial because of a lack of strong prospective results. An ongoing prospective trial from M.D. Anderson Cancer Center (ClinicalTrials.gov registration NCT03161522), comparing chemoradiation with or without surgery to systemic therapy alone for esophageal or gastric cancer with oligometastases may provide further insight into the value of aggressively treating metastatic lesions. With the advent over the past years of more effective and tolerable systemic therapy and technological advances in surgical therapy and radiation therapy, retrospective findings supported an aggressive local therapy in patients with limited metastatic disease. This present study showed an improvement in dysphagia score, an increase in PFS and a trend toward longer OS in esophageal cancer patients with ≤3 metastases who received concurrent chemoradiation, we recommend that aggressive local therapy be further tested in larger, prospective studies in which overall survival is the primary endpoint to define which subgroups of patients are most likely to benefit.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (No. U1604175).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Medical Ethics Review Committee of Renmin Hospital of Wuhan University, and the Number was 2017K-C023, and informed consent for participation was exempted by the board due to its retrospective nature.
References
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, , based on November 2015 SEER data submission, posted to the SEER web site, April 2016. Accessed November 10, 2016.http://seer.cancer.gov/csr/1975_2013/
- NCCN clinical practice guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers (2017.V4).
- Guttmann DM, Mitra N, Bekelman J, et al. Improved overall survival with aggressive primary tumor radiotherapy for patients with metastatic esophageal cancer. J Thorac Oncol 2017;12:1131-42. [Crossref] [PubMed]
- Saddoughi SA, Reinersman JM, Zhukov YO, et al. Survival after surgical resection of stage IV esophageal cancer. Ann Thorac Surg 2017;103:261-6. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC. editors. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2009, 103-15.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- O’Rourke IC, Tiver K, Bull C, et al. Swallowing performance after radiation therapy for carcinoma of the esophagus. Cancer 1988;61:2022-6. [Crossref] [PubMed]
- Institute NC. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2009. Available online: (cited 2017 April 1).http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53. [Crossref] [PubMed]
- Jingu K, Umezawa R, Matsushita H, et al. Chemoradiotherapy for T4 and/or M1 lymph node esophageal cancer: experience since 2000 at a high-volume center in Japan. Int J Clin Oncol 2016;21:276-82. [Crossref] [PubMed]
- Penniment MG, De Ieso PB, Harvey JA, et al. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in advanced oesophageal cancer: a multicentre randomised controlled trial (TROG 03.01). Lancet Gastroenterol Hepatol 2018;3:114-24. [Crossref] [PubMed]
- Akl FM, Elsayed-Abd-Alkhalek S, Salah T. Palliative concurrent chemoradiotherapy in locally advanced and metastatic esophageal cancer patients with dysphagia. Ann Palliat Med 2013;2:118-23. [PubMed]
- Suzuki G, Yamazaki H, Aibe N, et al. Palliative radiotherapy in the local management of stage IVB esophageal cancer: factors affecting swallowing and survival. Anticancer Res 2017;37:3085-92. [PubMed]
- Ikeda E, Kojima T, Kaneko K, et al. Efficacy of concurrent chemoradiotherapy as a palliative treatmentin stage IVB esophageal cancer patients with dysphagia. Jpn J Clin Oncol 2011;41:964-72. [Crossref] [PubMed]
- Mckeman M, Mcmillan DC, Anderson JR, et al. The relationship between quality of life (EORTC QLQ-C30) and survival in patients with gastro-oesophageal cancer. Brit J Cancer 2008;98:888-93. [Crossref] [PubMed]
- Hingorani M, Dixit S, Johnson M, et al. Palliative radiotherapy in the presence of well-Controlled metastatic disease after initial chemotherapy may prolong survival in patients with metastatic esophageal and gastric Cancer. Cancer Res Treat 2015;47:706-17. [Crossref] [PubMed]