
The donor heart and organ perfusion technology
Introduction
Extra-corporeal organ perfusion is an area gaining traction in transplantation, with increasing evidence showing superiority over cold storage of donor organs, and the potential for organ recovery and repair. This technology is currently utilised in the procurement of heart, lungs, liver and kidneys with varying outcomes. This review will focus on the current progress in device technology for heart transplantation.
A history of heart transplant
The first heart transplant was performed in 1967 by Dr. Christiaan Barnard, in Groote Schuur Hospital, Cape Town (1,2). The donor was a young lady who sustained irrecoverable traumatic brain injury; and the recipient, a 55-year-old man with ischaemic cardiomyopathy. In the absence of brain-death legislation at the time, withdrawal of life support and subsequent rapid procurement of the donor heart upon asystolic arrest occurred in an adjacent operating room to that of the recipient’s transplant procedure. Whilst the recipient only survived 18 days, this was the ‘proof-of-concept’ that resulted in widespread adoption of heart transplantation globally. However, early results were universally poor, resulting in the abandonment heart transplantation by many units. It was not until the 1980’s when there was a revival of heart transplantation with the introduction of brain death legislation (3), clinical acceptance of criteria to establish brain-stem death and more importantly, the discovery of cyclosporin for the immunosuppressive therapy for acute graft rejection.
Today, heart transplantation remains the gold standard treatment for end stage heart failure refractory to medical therapy with reported 1-year survival of 85–93%, long-term survival up to 69% at 10 years (4,5). Despite the increase prevalence of heart failure, transplant numbers have not seen a comparable rise. This is due to a shortage of donor hearts which can be attributed to improved road safety, continued low organ donation rates and increasingly older donors with multiple co-morbidities. Whilst, raising awareness for organ donation has shown an improvement in overall donation numbers, there remains a high demand with growing transplant wait-lists leading to the necessary expansion of current acceptable donor criteria (marginal criteria) and inclusion of a new category of organ donors, donation after circulatory death (DCD) (5-8). Whilst the small initial historical cohort of heart transplants were from this DCD pathway, the donors and matched recipients were in these instances co-located in adjacent operating theatres. This practice was subsequently abandoned due to the logistics of donor transfer to the recipient hospital and with the adoption of long-distance organ procurement with cold static preservation of organs during transportation.
One of the biggest concerns associated with the use of these marginal or extended-criteria organs is the unknown risk of graft failure and graft dysfunction post implantation. This is particularly pertinent in the DCD cohort, as these donor hearts have been exposed to an inevitable period of warm ischaemia prior to the institution of cardio-protective solution. The advent of ex-situ machine perfusion (MP) allows these organs to be reanimated and assessed prior to consideration for transplantation. The use of MP has led to the first successful distant procurement DCD heart transplantation in 2014 by Dhital and co-workers from St Vincent’s Hospital, Sydney, Australia (9). To date, 100 cases of DCD heart transplants has been performed across 6 units in Australia, the UK and Belgium.
MP and heart transplantation
The concept of ex-situ perfusion has long been desired and is potentially the ideal preservation strategy for donor organ management. Stemming from Langendorff’s isolated heart perfusion model in mammals (10), which has been paramount in the study of ischaemic reperfusion injury (IRI) and heart transplantation, the recent development of clinical MP devices has been long in the making. The advantages of MP are derived largely from its ability to provide continuous circulation to the organ thereby protecting it from ongoing ischaemic injury. Furthermore, it provides a platform for strategies to mitigate reperfusion injury, detect anatomical abnormalities, uncover physiological dysfunction and ‘recover’ borderline organs through pharmacotherapy.
Currently, cold static storage (CSS) of donor heart remains the standard practice in most transplant units (11). The donor heart is flushed with preservation solution in-vivo, rapidly explanted and stored in cold saline or preservation flush solution at 4 °C. The organ is expediently transferred to the recipient hospital for implantation. Hearts procured in this method have a tolerable ischaemic period between 4–6 h, after which the organ deterioration leads to higher risk of graft failure and poorer outcomes in the recipient. Furthermore, using the current method of cardiac preservation, the donor heart is assessed in-vivo with no further evaluation once the heart has arrested and is stored, discounting any occult disease or injury that may have occurred during the retrieval process or in transit. Despite this, CSS hearts have shown consistent outcomes and is a reliable and cost-effective method of organ procurement particularly when there is no suspicion of organ marginality.
In contrast, the use of MP in the procurement of donor heart is currently limited to a few centres in the United States and Europe. At present, there is only one device available for clinical use, which is the Transmedic Organ Care System (OCS) Heart. This system uses a combination of donor blood and a proprietary solution as perfusate for the heart at mild hypothermia (34 °C). The benefit of normothermic perfusion is its ability to reanimate the heart and allow the clinician to perform functional assessment of the donor heart. At present, a combination of visual inspection, metabolic and flow parameters are used to determine organ viability. As oppose to the stringent time limitation in CSS, normothermic machine perfusion (NMP) has enabled clinicians to logistically delay implantation time with no significant consequences (12).
Role of MP
Key benefit of MP is the ability to maintain perfusion of the heart, meeting the metabolic needs of tissues and limiting progression towards irreversible cellular injury and death. In the marginal donation after brain death (DBD) setting, donor haemodynamics are managed carefully until the time of procurement, thereby limiting the duration of tissue ischaemia. Preservation flush is rapidly administered upon cross-clamping of the aorta to arrest cardiac activity, reducing the risk of ischaemic injury. The heart is rapidly explanted and installed on the device and flow is restored.
The reanimated heart now receives antegrade coronary perfusion via retrograde filling from the aorta. Coronary effluent drains from the coronary sinus into the right atrium and follows the normal path of circulation into the right ventricle before being ejected into the cannulated pulmonary artery and then into the circuit reservoir. The device permits measurements of the following physiological parameters: ECG, haematocrit, SaO2, aortic flow, aortic pressure, coronary flow and temperature. Serial sampling from the arterial and venous ports of the circuit permits routine blood gas measurements including lactate levels. The target physiological and biochemical parameters are presented in Table 1.
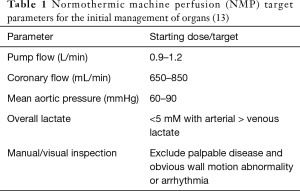
Full table
Once flow and pressure on NMP have stabilised, the heart is periodically assessed with arterial and venous blood lactate measurements. Current recommendation by Transmedics OCS Heart are presented in Table 1. The heart is considered feasible for transplantation upon reaching the target lactate parameters and if there are no gross abnormalities on visual inspection. The system allows for epicardial echocardiography and is also compatible for coronary angiography if required (14).
Standard brain-dead donors (DBD)
As discussed earlier, only one system utilising normothermic perfusion is currently available for clinical use. To date, more than 200 DBD and 100 DCD heart transplants have been performed using the OCS system (see Table 2). The use of OCS in the DBD setting has been extensively discussed previously by Messer et al. (15). To date, the PROCEED II and PROTECT II trial has shown non-inferiority when comparing NMP to CSS using the Transmedic OCS Heart.

Full table
Marginal brain-dead donors (MBD)
MBD or extended criteria brain dead donor hearts have been utilised since 2000, with comparable survival to DBD as reported by multiple single centre studies (5,16-18). It is important to note however, that in these studies, the hearts were cold stored after procurement without the use of NMP. Furthermore, the inclusion and exclusion criteria were different in each study accounting for the variability in outcomes and is a key limitation to its wider application. Despite these shortcomings these studies suggest that hearts from older donors with mild ventricular impairment are feasible for transplantation.
When comparing the role of NMP in the recovery of MBD hearts, there are currently no comparison studies published. Single centre studies to date have shown comparable survival to DBD (19-21) where the key advantage of NMP is to eliminate the risk of prolonged ischaemic time thereby allowing access to farther donors which would have otherwise been refused.
The EXPAND study is the only multicentre study comparing CSS and NMP in MBD donors. This is currently an ongoing study with a projected completion date in mid-2019.
DCD donors
DCD donors are a new category of heart donors. In contrast to DBD donors, DCD donor hearts are exposed to prolonged periods of warm ischaemia, and as a result require functional assessment prior to transplantation. As the donor undergoes the process of circulatory arrest, these hearts exhibit severe right atrial and ventricular distension (22); furthermore, the period of warm ischaemia may result in irreversible myocardial injury. Iyer et al. have shown that porcine hearts exposed to more than 20 minutes of circulatory arrest is associated with irreversible cardiac injury, and through pharmacological conditioning this time-frame can be prolonged to 30 minutes (23).
In this category of donors, post asystolic functional assessment of the hearts is necessary. Currently, two methods of post-asystolic assessment and procurement are practiced. Figure 1 outlines the major process in both methods of organ retrieval and recovery.
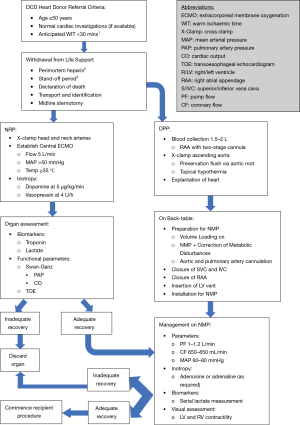
Sydney direct procurement protocol (DPP) (7,24)
Once death is confirmed, the donor is rapidly transferred to the operating room for organ procurement. 1.2–1.5 L of donor blood is collected centrally, followed by delivery of cardio-protective solution via the aortic root. The donor heart is topically cooled and explanted upon completion of the preservation flush. The heart is instrumented for NMP. Upon reperfusion, any arrhythmia and electrolyte imbalances are corrected. Coronary flow and aortic pressure targets are met by titrating aortic flow, adrenaline and adenosine infusions.
Papworth normothermic regional perfusion (NRP) (24,25)
The donor is expediently bridged onto central extracorporeal membrane oxygenation (ECMO) after death ensues, with cross-clamping of the arch vessels to prevent cerebral perfusion. ECMO flow is maintained at 5 L/min, with a target MAP >50 mmHg. The organs are resuscitated for a short period, after which a combination of biomarkers and functional assessment is performed. Hearts that demonstrate adequate recovery are flushed with cardioplegia solution of choice and following explanation is installed onto NMP for transportation.
Management of DCD hearts on NMP is largely similar to DBD hearts procured using NMP. Of note however is the importance of correcting any electrolyte imbalances in the perfusate, especially in the DCD group, which usually demonstrate significant acidosis, hyperkalaemia and hypercalcemia. Furthermore, in contrast to Transmedic’s recommendation to commence high dose of adenosine infusion in the DBD setting, within the DCD cohort, we have noted increase adrenaline requirement to counter a vasodilated coronary circulation with high coronary flow and a low perfusion pressure immediately post-reperfusion.
To date, 100 cases of DCD heart transplantation has been performed over 6 centres between Australia, United Kingdom and Belgium. Current published outcome by the Papworth experience reported a comparable short term survival between the DCD and DBD cohorts with a 1-year survival of 86% in the DCD-DPP group, and 100% in the DCD-NRP arm vs. 96% in DBD group; ECMO use was reported at 12% (8). In Australia, we have performed 27 cases of DCD heart transplantation using DPP with only one mortality (96% survival); ECMO support post-transplant was 30% (n=8). Despite the excellent outcomes to date, uptake of DCD transplantation remains low due to the lack of more rigorous physiological and biochemical metrics, lack of surgical experience with MP and inability to fully assess ventricular function in working modality in the current state of the device.
Pre-clinical devices
Apart from the Transmedic OCS Heart™ which is currently used clinically, four other cardiac perfusion devices have been trialled in animal studies with good outcomes. All four devices utilise hypothermic machine perfusion (HMP) at 4 °C. These devices are listed in Table 3.

Full table
HMP vs. NMP
While there are no studies comparing HMP to NMP in cardiac allograft preservation, both preservation techniques have demonstrated improved or comparable outcomes to CSS hearts. Current evidence suggests that the provision of oxygen renders better protection to cardiomyocytes, preserves cellular ATP stores and better maintains membrane conditions (34). Furthermore, the continuous perfusion prevents build-up of toxic metabolites (i.e., lactate and adenosine) which may contribute to poor ventricular function or serve as a substrate for the generation of free radicals upon reperfusion of the organ (29). Hearts preserved using HMP is maintained in the arrested state.
As oppose to NMP, due to the lower temperatures, sufficient oxygen delivery can be achieved using oxygenated crystalloid solution. The resulting cardiac oedema does appear to be readily reversible post-transplantation and has not been shown to affect detrimentally cardiac function (35). Furthermore, both systems currently lack a formal and objective method for functional assessment of the allograft. As currently used biomarker (i.e., lactate) does not necessarily reflect the functional ability of the organ (24), a more reliable marker or mode of assessment is needed.
Future of organ perfusion and future directions
The changing landscape of heart transplantation with greater utility of high-risk donor organs to address the issue of organ shortage has led to more stringent assessment of donor hearts. The use of machine technology has allowed a 15–20% increase in transplant volumes by utilising hearts that were otherwise rejected. While the current NMP system is not without a significant price tag, overall cost saving through reduced hospital readmissions for management of decompensated heart failure may justify and promote greater use of organ perfusion systems (36). Further cost-benefit evaluation is required and may need case by case assessment.
In regard to HMP versus NMP, whether maintaining the heart in the arrested state will provide better protection is a moot point, which leads to the most important issue being the inability for either systems to perform functional assessment in their current design configuration. This is especially pertinent when considering MBD or DCD hearts where adequate cardiac recovery is questionable. Regardless, the technology is here to stay not only for improved organ preservation and assessment, but also for extra corporeal therapy such as immune-modulation and surgical intervention for valvular and coronary lesions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brink JG, Hassoulas J. The first human heart transplant and further advances in cardiac transplantation at Groote Schuur Hospital and the University of Cape Town. Cardiovasc J Afr 2009;20:31-5. [PubMed]
- Hassoulas J. Heart transplantation: research that led to the first human transplant in 1967. S Afr Med J 2011;101:97-101. [Crossref] [PubMed]
- Sade RM. Brain death, cardiac death, and the dead donor rule. J S C Med Assoc 2011;107:146-9. [PubMed]
- Wilhelm MJ. Long-term outcome following heart transplantation: current perspective. J Thorac Dis 2015;7:549-51. [PubMed]
- Chew H, Lo P, Cao J, et al. Retrospective Single Centre Comparison of Outcomes between Standard Criteria and Marginal Criteria Brain Dead Heart Transplantation. J Heart Lung Transplant 2016;35:S296. [Crossref]
- Bombardini T, Arpesella G, Maccherini M, et al. Medium-term outcome of recipients of marginal donor hearts selected with new stress-echocardiographic techniques over standard criteria. Cardiovasc Ultrasound 2014;12:20. [Crossref] [PubMed]
- Dhital KK. Chew HCMacdonald PS. Donation after circulatory death heart transplantation. Curr Opin Organ Transplant 2017;22:189-97. [Crossref] [PubMed]
- Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2017;36:1311-8. [Crossref] [PubMed]
- Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet 2015;385:2585-91. [Crossref] [PubMed]
- Zimmer HG. The Isolated Perfused Heart and Its Pioneers. News Physiol Sci 1998;13:203-10. [PubMed]
- Monteagudo Vela M. Sáez DGSimon AR. Current approaches in retrieval and heart preservation. Ann Cardiothorac Surg 2018;7:67-74. [Crossref] [PubMed]
- Stamp NL, Shah A, Vincent V, et al. Successful Heart Transplant after Ten Hours Out-of-body Time using the TransMedics Organ Care System. Heart Lung Circ 2015;24:611-3. [Crossref] [PubMed]
- Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015;385:2577-84. [Crossref] [PubMed]
- Anthony C, Michel J, Christofi M, et al. Ex Vivo Coronary Angiographic Evaluation of a Beating Donor Heart. Circulation 2014;130:e341-3. [Crossref] [PubMed]
- Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transpl Int 2015;28:634-42. [Crossref] [PubMed]
- Samsky MD, Patel CB, Owen A, et al. Ten Year Experience with Extended Criteria Cardiac Transplantation. Circ Heart Fail 2013;6:1230-8. [Crossref] [PubMed]
- Rajagopalan N, Dennis DR, Ross HL, et al. Utilizing Expanded Donor Selection Criteria for Heart Transplantation: A Single Center Experience. J Heart Lung Transplant 2017;36:S42-3. [Crossref]
- Lima B, Rajagopal K, Petersen RP, et al. Marginal cardiac allografts do not have increased primary graft dysfunction in alternate list transplantation. Circulation 2006;114:I27-32. [Crossref] [PubMed]
- García Sáez D, Zych B, Sabashnikov A, et al. Evaluation of the Organ Care System in Heart Transplantation With an Adverse Donor/Recipient Profile. Ann Thorac Surg 2014;98:2099-105. [Crossref] [PubMed]
- Yeter R, Pasic M, Hübler M, et al. Extended donor criteria in heart transplantation: 4-year results of the experience with the Organ Care system. Thorac Cardiovasc Surg 2014;62:SC44. [Crossref]
- Chew H, Cheong C, Fulton M, et al. Outcome After Warm Machine Perfusion (WMP) Recovery of Marginal Brain Dead (MBD) and Donation After Circulatory Death (DCD) Heart Transplantation. J Heart Lung Transplant 2017;36:S45-6. [Crossref]
- Iyer A, Chew HC, Gao L, et al. Pathophysiological Trends During Withdrawal of Life Support: Implications for Organ Donation After Circulatory Death. Transplantation 2016;100:2621-9. [Crossref] [PubMed]
- Iyer A, Gao L, Doyle A, et al. Increasing the Tolerance of DCD Hearts to Warm Ischemia by Pharmacological Postconditioning. Am J Transplant 2014;14:1744-52. [Crossref] [PubMed]
- White CW, Messer SJ, Large SR, et al. Transplantation of Hearts Donated after Circulatory Death. Front Cardiovasc Med 2018;5:8. [Crossref] [PubMed]
- Messer SJ, Axell RG, Colah S, et al. Functional assessment and transplantation of the donor heart after circulatory death. J Heart Lung Transplant 2016;35:1443-52. [Crossref] [PubMed]
- Steen S, Paskevicius A, Liao Q, et al. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand Cardiovasc J 2016;50:193-200. [Crossref] [PubMed]
- Nilsson J, Jernryd V, Qin G, et al. Non Ischemic Heart Preservation. J Heart Lung Transplant 2018;37:S13. [Crossref]
- Van Raemdonck D, Rega F, Rex S, et al. Machine perfusion of thoracic organs. J Thorac Dis 2018;10:S910-23. [Crossref] [PubMed]
- Rosenbaum DH, Peltz M, DiMaio JM, et al. Perfusion Preservation versus Static Preservation for Cardiac Transplantation: Effects on Myocardial Function and Metabolism. J Heart Lung Transplant 2008;27:93-9. [Crossref] [PubMed]
- Peltz M, Brant S, Cobert M, et al. Continuous Coronary Sinus Perfusion for Recovery of Hearts from Non-Heart-Beating Donors. Am J Transplant 2013;13(suppl 5). Available online: https://atcmeetingabstracts.com/abstract/continuous-coronary-sinus-perfusion-for-recovery-of-hearts-from-non-heart-beating-donors/, Accessed March 10, 2019.
- Cobert ML, Merritt ME, West LM, et al. Metabolic Characteristics of Human Hearts Preserved for 12 Hours by Static Storage, Antegrade Perfusion or Retrograde Coronary Sinus Perfusion. J Thorac Cardiovasc Surg 2014;148:2310-5.e1. [Crossref] [PubMed]
- Van Caenegem O, Beauloye C, Vercruysse J, et al. Hypothermic continuous machine perfusion improves metabolic preservation and functional recovery in heart grafts. Transpl Int 2015;28:224-31. [Crossref] [PubMed]
- Michel SG, La Muraglia GM 2nd, Madariaga ML, et al. Twelve-Hour Hypothermic Machine Perfusion for Donor Heart Preservation Leads to Improved Ultrastructural Characteristics Compared to Conventional Cold Storage. Ann Transplant 2015;20:461-8. [Crossref] [PubMed]
- Cobert ML, West LM, Jessen ME. Machine perfusion for cardiac allograft preservation. Curr Opin Organ Transplant 2008;13:526-30. [Crossref] [PubMed]
- Macdonald PS, Chew HC, Connellan M, et al. Extracorporeal heart perfusion before heart transplantation: the heart in a box. Curr Opin Organ Transplant 2016;21:336-42. [Crossref] [PubMed]
- Jain P, Prichard RA, Connellan MB, et al. Long distance heart transplantation: a tale of two cities. Internal Medicine Journal 2017;47:1202-5. [Crossref] [PubMed]


