
Diagnostic and clinical features of lung cancer associated with cystic airspaces
Introduction
Lung cancer remains the leading cause of cancer-related deaths worldwide (1), generally presenting on imaging studies as mass or nodule. “Lung cancer associated with cystic airspaces” is a rarer manifestation, in which lung cancer presents on CT as a “cystic area” with associated consolidation and/or ground glass. Since the first publication in the 1940’s (2), numerous cases have been reported in different ways e.g., “Carcinoma of the bronchus presenting as thin-walled cysts” (3), “Carcinoma masquerading as a thin walled cyst” (4), “Bronchogenic carcinoma and giant bullous disease” (5), “Bronchioloalveolar carcinoma arising in longstanding lung cysts” (6), “Lung carcinoma associated with bullous lung disease” (7).
Upon today, there is no clear definition of what this cystic component is, nor is there one clear diagnosis on histopathology. The appearance of lung cancer associated with cystic airspaces on CT is that of a single or multiple “cystlike” components, with areas of ground glass or consolidation abutting the wall of the cystic part or interspersed between the cystic components. The Glossary of Terms for Thoracic Imaging by the Fleischner Society defines “cyst”, but currently does not define this lung cancer entity (8). Despite the absence of a clear definition, it should not be confused with or misinterpreted as cavitary lesion. A cyst contains air, but occurs in an area with normal lung and has a thinner wall, less than 2 mm (8). A cavity is a gas-filled space, seen as a lucency or low-attenuation area, within pulmonary consolidation, a mass or a nodule (8). In contrast to cavities or cavitary lung masses which can sometimes contain a fluid level, the cystic part in lung cancer associated with cystic airspaces does not contain fluid. In cases where the diffuse wall thickening of a cystic airspace tumor progresses (type III as further discussed), it may however mimic a cavitary lung lesion.
Although overall still rare, lung cancer associated with cystic airspaces is becoming more frequently encountered on imaging studies, due to the widespread use of CT in daily clinical practice and evolution of lung cancer screening programs. Unfortunately, due to the uncommon morphology lung cancer associated with cystic airspaces often remains unrecognized with risk of delayed diagnosis (9,10). Whereas early lung cancers most often present as subsolid or solid nodules (11-15), less common manifestations have been identified, including manifestation as a lesion with cystic airspace morphology (16,17). In a screensetting, Farooqi et al. observed a 3.7% of cancer cases presenting with cystic airspace. With an incidence of 3.5%, Kaneda et al. reported similar findings in a surgical, predominant non-screen, population (18). In the largest study by Fintelmann, 1% of lung cancer patients showed this morphological tumor type on CT-imaging studies (19). This 1% is probably underestimated since a high number of cases (325 cases) were excluded because of an observation period of less than 6 months.
Clinical characteristics
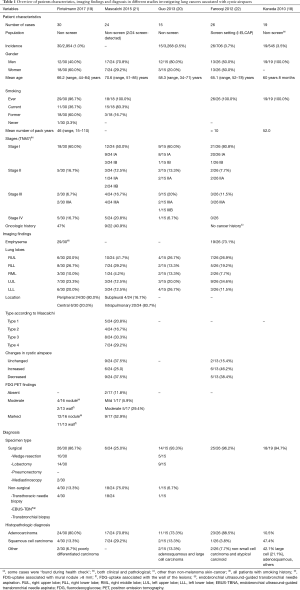
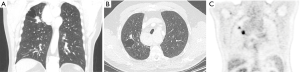
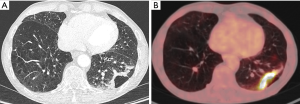
Guo (20), Mascalchi (21) and Kaneda (18) et al. showed that lung cancer associated with cystic airspaces was more frequent in their series in men than in women. Farooqi et al. did show however in a screensetting that 50% of this tumor type occurred in men and 50% in women (22). The largest study by Fintelmann et al. showed a higher incidence of female patients (19) (Table 1). Mean age at time of diagnosis varied in the different studies from 58.3 to 70.6 years. Except for one never-smoker in the study by Fintelmann, the majority of cases were ever (former and current) smokers. This is also reflected in the high rate of patients presenting with emphysematous changes on imaging in all studies (18-22). Fintelmann et al. showed an association of lung cancer associated with cystic airspaces with smoking and presence of emphysema (19). The association with smoking status is also reflected in the relatively higher incidence of this tumor type (in comparison to the other studies) in the lung cancer screening population reported by Farooqi et al. (Table 1) (22). The studies from Fintelmann and Mascalchi showed that respectively 47% and 41% of patients with a cystic airspace tumor had a previous cancer history, often of pulmonary origin. Related to the screensetting, patients in the study by Farooqi were asymptomatic. The study by Fintelmann does not give insight in the clinical presentation. One might expect a similar clinical presentation as with other lung cancer types, depending on the stage of tumor spread. In rare cases, acute chest pain due to a pneumothorax can be the initial clinical presentation in a patient with a cystic airspace tumor. (Figure 1).
Full table
Pathogenesis
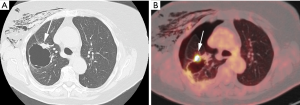
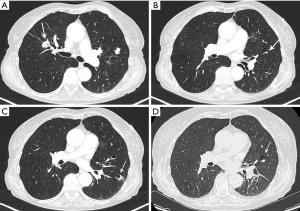
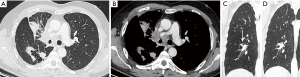
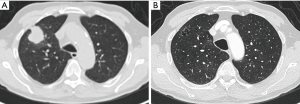
The exact carcinogenic mechanism remains unclear. A number of possible hypotheses have been discussed in literature. The first reports in the 1940’s assigned the cause of lung cancer associated with cystic airspaces to a previously existing congenital malformation (2). Anderson and Pierce described in the 1950’s a series of 6 cases of carcinoma of the bronchus in which the radiological appearance was that of a thin-walled cavity or cyst. They suggest that this was not caused by a spreading consolidation that later excavated, but rather by a thin layer of malignant cells initially growing along a pre-existing cavity that resulted from intermittent valvular bronchial occlusion caused by a small nodule (3). Different authors address the relationship of lung carcinoma with bullous disease of the lung (5,7,23). In the majority of case reports, the exact type of the cyst is not specified. Maki et al. described a series of 20 patients with CT-appearances of bronchogenic carcinoma associated with bullous lung disease. Lantuejoul et al. described 7 cases of (formerly called) congenital cystic adenomatoid malformation (CCAM) type I with adenocarcinoma. They showed that mucinous proliferations in type I CCAM harbor the same differentiation profile than corresponding mucinous bronchioloalveolar carcinoma of the lung (24). A review by Kaneda et al. of 95 cases (predominantly of Asian origin) stipulated that limited air flow in areas of compressed parenchyma and connective tissues surrounding a pulmonary bulla may cause deposits of microorganisms on the wall of the bulla with repeated infection. This repeated inflammatory process might cause a fibrous scar to form around the bulla, causing accumulation of carcinogens. Cysts may interfere with ventilation and lung clearance and thus facilitate deposition of carcinogens (2,5). The study by Farooqi et al. showed that pathologic findings reflected the CT-imaging features with large dominant cystic airspace with associated carcinoma, but that the histological features of the cystic lesions were variable. There was however no evidence for any relationship with pre-existing congenital cystic lung disease (22). In the series of Mascalchi et al., 6 out of 24 lesions showed an increase in size of the cystic airspace, which was presumably caused by a valve mechanism on the distal airway connected with the cystic airspace (21). Fintelmann et al. also observed a change in morphology from unilocular to multilocular in 6 out of 24 cases. In 16 cases, the size of the cystic component increased (19). This study gives more insight in the pathogenesis: the cystic airspace on CT may be caused by different mechanisms. Most commonly (in 38% of cases) this is caused by a check-valve mechanism obstructing the small airways. Other causes include lepidic growth of adenocarcinoma on emphysematous lung parenchyma, cyst formation of tumor and growth along the wall of a pre-existing bulla. The fact that lung cancer associated with cystic airspaces has also been described in synchronous (Figure 2) and metachronous (Figure 3) cases, suggests an etiology related to intrinsic mechanism of tumor growth in some cases, rather than developmental changes in pre-existing cystic or bullous lung disease (10,21).
Histopathology and mutational analysis
Most studies revealed adenocarcinoma as being the predominant histological type in lung cancers associated with cystic airspaces with up to 80% and 88% in the largest studies (by Fintelmann and Farooqi) (19,22). Data on different subtypes of adenocarcinoma is scarce: in the study by Fintelmann, 6 out of 21 cases presented with lepidic growth of adenocarcinoma (19). In the series of Guo, three patients with adenocarinoma (3/11) presented with adenocarcinoma in situ (AIS) and 2 (2/11) with minimally invasive adenocarcinoma (MIA) (20). Second most common is squamous cell carcinoma. Other tumor types have also been reported (Table 1). Data on mutational analysis is limited. Guo et al. found an epidermal growth factor receptor (EGFR) mutation in 3 out of 8 patients from Asian origin. Two patients (2/3) had an EGFR exon 19 deletion, 1 an exon 21 mutation (L858R). One patient developed a T790M mutation. The study by Fintelmann is the largest to give insight in mutational analysis: KRAS mutations were found as being the predominant alterations. With an occurrence of 54% of all tumors and 64% of adenocarcinoma this is higher than one might expect in a non-small cell lung cancer (NSCLC) tumor population without cystic airspace morphology (Table 1) (19).
A recent study by Toyokawa et al. investigated the occurrence of programmed death-ligand 1 (PD-L1) expression in “emphysematous bullae-associated lung adenocarcinomas”: 58% of ‘cancer adjoining bulla’ cases in this study of 369 patients showed expression of PD-L1 (cut-off value set at 5%). This frequency was higher than found in the group of only ‘emphysematous bulla related lung adenocarcinomas’ (44.4%) and the group of adenocarcinomas not related to any emphysematous changes (11.8–12.9%) (25). The high incidence in this study of ‘cancer adjoining cystic airspaces’ (50/369–13.5%) makes it unclear to what amount these cases resemble the same entity as lung cancer associated with cystic airspace in other studies. Although data is limited, findings may indicate that molecular profiling is mandatory in patients presenting on CT-imaging as lung cancer associated with cystic airspaces.
CT-findings and classification
As previously mentioned, the term ‘Lung cancers associated with cystic airspaces’ represents a morphologically heterogeneous group of lesions in which a primary lung cancer manifests on CT with a ‘cystic’ component or ‘cystic airspace(s)’. In 2006 Maki et al. were the first to describe a classification system for lung cancers associated with cystic airspaces (26). This system was later modified by Mascalchi and recently by Fintelmann and colleagues (Figure 4). Mascalchi et al. describe a classification system with four morphologic subtypes (Figure 4A) (21). In Type I there is a nodule abutting the cyst wall (Figures 1A,2D,3E,5). In Type II, the nodule also arises from the cyst wall but projects in the lumen of the cyst (Figure 3A). In Type III there is a soft tissue density extending along the wall of the cystic airspace or more diffuse wall thickening (Figure 6). In type IV, the lesion has a more ‘multicystic’ aspect with areas of consolidation interspersed with cystic areas (Figure 7). Based on the initial scheme of Mascalchi, Fintelmann and colleagues recently developed a new classification system with a number, uppercase and lowercase letter (Figure 4B). A number is given for the airspace morphology: “0” in case of a thin-walled cyst, number “1” for an endophytic nodule, number “2” for an exophytic nodule and number “3” for a thick-walled cystic airspace. The uppercase letter describes the density/consistency of the lesion: “S” for solid, “NS” for pure ground glass or nonsolid and “PS” for part solid. The 3rd evaluation is that of the cystic airspace (with lowercase letters): “u” for unilocular and “m” for multilocular lesions.
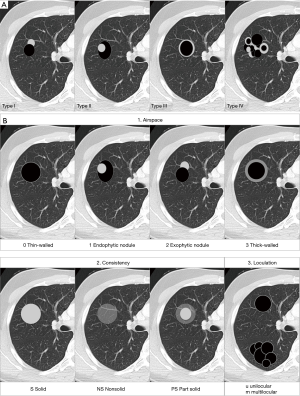
The value of these classification systems still needs to be defined: currently there is no available data on prognosis and/or survival related to specific categories. These classification systems may however be valuable in future studies and may allow correlation of more data of specific types with molecular profile, evolution, management and prognosis.
All studies show a uniform distribution of tumors throughout the lung, with no predilection for any lobe. Within a lobe, lesions are preferably located in the periphery (Table 1). This is not surprising, since the majority of lesions were adenocarcinoma and these tend to be found in a more peripheral location. In the study by Mascalchi, type III and IV are most common and Type II occurs less frequent. Since morphological findings and changes during follow-up in type I, II and III can be subtle and lesions may become more complex, a CT protocol with thin-section images is advised. Chest CT scans should be reconstructed with contiguous thin sections (≤1.5 mm, preferably 1.0 mm). Multiplanar reconstructions in the coronal and sagittal plane can be recommended to better delineate the relationship between the cystic and non-cystic part of the tumor and to better characterize the lesion. Archiving of thin-section CT-images is mandatory for accurate follow-up. Furthermore, access to previous imaging studies can be of utmost importance for correct (early) diagnosis.
18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) imaging
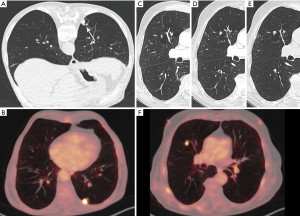
There is only limited data concerning PET-findings. In 70% of patients in the study by Fintelmann, 18F-FDG-PET was available. In the majority of lesions, uptake was noted both in the wall and in a mural nodule. Regarding the mural nodule, uptake was moderate in 4 cases and marked in 12 cases. In 2 cases the wall showed moderate uptake and in 11 cases marked. Mural nodules <8 mm did not show FDG-uptake. Wall thickening without mural nodule only showed uptake in cases with a solid component of more than 8 mm (19). The study of Mascalchi showed marked uptake in the majority of cases. Cases in the study by Mascalchi that did not show uptake on PET, were all adenocarcinoma, with predominant Type I and II morphology. Cases with marked uptake were type III and IV lesions (21).
Whereas PET-CT (Figures 1B,3A,3F,5C) plays an important role in the diagnostic work-up of suspicious pulmonary nodules and lung cancer staging, its role in diagnosis and management of cancers associated with cystic airspaces is less evident. Cystic lesions with a thin wall and small mural nodule, may not show uptake. In Mascalchi Type III and IV lesions, the uptake may be altered by the cystic changes interspersed with the areas of consolidation. As discussed, the majority of histological types associated with this entity are adenocarcinoma. Depending on the amount of lepidic growth and/or ground glass component on imaging, uptake on PET may be reduced and even absent (27-29). Correlation of findings on PET with morphology on CT is crucial in lesions with associated cystic airspace(s): absence of uptake does not rule out a tumor. On the contrary, in a lesion showing uptake, a benign etiology (infectious, inflammatory) should also be taken into account.
Natural evolution and prognosis
The evolution of the cystic airspace is variable: it may stay unchanged, increase or decrease in size and the type of lesion may change over time (Table 1). Of the 26 cases from the International Early Lung Cancer Action Program (I-ELCAP) study by Farooqi et al., half of them (13/26) were detected at baseline and the other half during annual follow-up. This finding may reflect a relatively aggressive nature. Cancers diagnosed during subsequent annual repeat screening showed wall thickening and development of a mural nodule, following a relatively uniform thin wall on the baseline study. The group of patients with a nodular component of the tumor on baseline scanning, showed a greater degree of wall thickening. This might also reflect a more aggressive growth pattern (22). In the study by Mascalchi et al., in the majority of patients the wall of the cystic airspace was initially thin becoming thicker over time.
The increase in tumor diameter resulted in decreased size of the cystic airspace component in most cases, giving rise to purely solid lesions with no residual cystic airspace in 5 out of 24 cases (21). It is therefore impossible to estimate the number of tumors that start as a lung cancer with cystic airspace, in particular in these cases where a solid tumor is observed at the time of diagnosis and no previous imaging is available (Figures 8,9). As some cystic airspaces appeared in a previously normal lung, the presence of a new cyst or cystlike lesions warrant attention and follow-up (19).
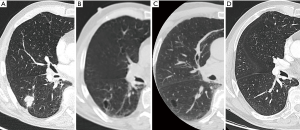
The risk of a cyst developing into a lung cancer or being the precursor of a lung cancer is still unknown. Data on this subject is scarce. Araki et al. investigated the incidence and natural course of pulmonary cysts in a cohort of participants of the Framingham Heart Study (30). Pulmonary cysts (not associated with emphysema) were found in 7.6% of the selected group, increasing with age: 4.9% at 40–49 years to 12.9% in participants 80 or older. Most of the cysts remained unchanged during longitudinal follow-up, some increased slightly in size. They do not describe any case associated with lung cancer formation. Guo et al. described a series of 321 patients undergoing surgery for ‘cystic’ lung disease, with 4.6% (15 cases) of these patients with cystic lung changes having lung cancer.
There is no prospective data on prognosis and survival. As one might expect from a screening setting, Farooqi et al. found 21 out of 26 cases being stage I (20 cases IA and 1 case IB), according to TNM7. Two cases were stage IIA and 3 cases stage IIIA but no stage IV was encountered (22). The studies with predominantly non-screen setting showed cases in different stages, mostly stage I but also including stage IV (Table 1). The retrospective study by Kaneda et al. on 19 patients who were surgically treated between 1998 and 2008 for ‘primary lung cancer adjoining pulmonary bulla’, showed a significantly worse survival curve in this small group of patients. In contrary to most studies, only 10.5% of cases were adenocarcinoma, the majority were squamous cell carcinoma (47.4%), with the others being large cell carcinoma, adenosquamous carcinoma and other histological types (42.1%) (18). From the 15 surgical patients of Guo et al. 13 were still alive at the end of the study, 2 patients (one stage IIIB and one stage IV) had died. Median follow-up period was 19.8 months (range, 3–38 months) (20). Fintelmann et al. suggested a negative effect on overall survival of this tumor type, related to the high number of cases with associated KRAS mutations. Two years after diagnosis, 41% of patients (12/29) in his series had died (19).
Differential diagnosis and pitfalls
Diagnosis of lung cancer associated with cystic airspaces can be challenging. Numerous benign causes may mimic the different types (Figure 10). A nodular solid morphology may point to a malignancy (irrespective of the associated cystic airspace), but type I and type IV lesions according to the classification of Mascalchi, may be prone to misinterpretation and delayed diagnosis. Imaging and clinical findings that allow discrimination between lesions with ‘a high likelihood of malignancy’ or ‘probably benign lesion’ are growth, medical history and clinical findings. Infection, inflammation and granulomatous diseases can all present with cyst-like or cavitated morphology with associated solid component or consolidation (Figure 10C) (31,32). In particular fungal disease with mycetoma formation in a previous thin walled cavitation or cystic airspace can mimic type II lesions (Figure 10D). Associated parenchymal findings such as nodules (with or without cavitation), consolidations, …may be more indicative of an infectious cause, rather than indicating a high risk of malignancy. Lung cancer associated with cystic airspaces can present with synchronous or metachronous lesions (as discussed), but this is rather unusual: so, multiplicity of lesions should point to a benign cause rather than malignancy. When numerous lesions are present, in particular with Mascalchi type III morphology, one might primarily consider cavitary lung disease with diffuse involvement, rather than multiple cystic airspace tumors (32). In case of a limited number of cystic airspace lesions, careful search for associated solid and/or subsolid nodules may indicate the possibility of malignant etiology (Figure 2). The role of 18F-FDG-PET to differentiate between benign and malignant cystic airspace lesions is very limited, since infectious (including fungal) diseases, inflammatory abnormalities and granulomatous diseases can also show high uptake (33,34).
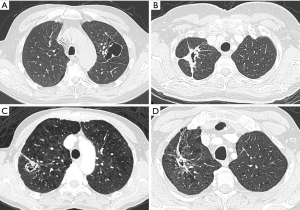
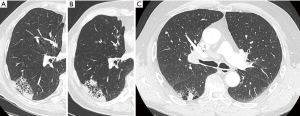
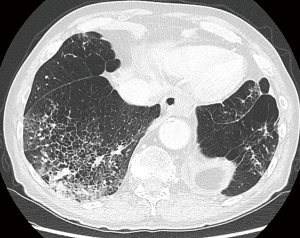
Time and evolution are crucial parameters for assessment. Lesions that persist over time or under therapy are less likely to be infection and close monitoring in these cases is warranted (Figure 7A,B). Previous imaging studies may indicate underlying cavitary or cystic disease, such as old tuberculosis cavern, pulmonary infarct sequelae, congenital cystic disease. Clinical status and previous medical history are important for management. Acute onset and illness in combination with abnormalities on imaging, may be more indicative of an infective cause. On the contrary, absence of acute illness and no significant previous medical history, may indicate that the cystic airspace lesion is likely to be malignant. Bullous lung disease and emphysematous changes make (early) diagnosis even more challenging, since these conditions predispose to both infection and malignancy (Figure 11) (31,32,35). Furthermore, underlying lung disease such as interstitial pneumonias or fibrotic lung disease can masquerade the presence of lung cancer, in particular when appearance is rather atypical (Figure 12) (36). Early cancer diagnosis in areas of distorted parenchyma is challenging (37,38). Correlation with clinical findings, comparison with previous CT studies and close monitoring are crucial in these cases.
Staging, management and screening
Whereas the majority of early lung cancers present as nodules, there is a wide variety of findings that represent early lung cancers, including lung cancers associated with cystic airspaces. The first study to discuss this entity in the era of lung cancer screening was the study by Farooqi et al. All lung cancer cases from the I-ELCAP lung cancer screening study were reviewed (22). On a total of 706 lung cancers (595 from baseline and 111 from annual screening), 26 cancers were identified as abutting the wall of a cystic airspace, comprising 3.7% of cancer cases. A retrospective study by Scholten et al. on the CT characteristics of interval and post-screen carcinomas in the Dutch-Belgian Lung Cancer Screening Trial (NELSON) showed that 22.7% (5/22) of missed cases presented as “a bulla with thickening of the wall”, corresponding to lung cancer associated with cystic airspaces (39).
Current lung nodule management guidelines, such as those from the British Thoracic Society and Fleischner Society, provide guidance for solid and subsolid nodules, but not for these tumors with cystic airspace (40,41). The current version of the Lung Imaging Reporting and Data System (Lung-RADS) has the possibility to upgrade lesions that have a suspicious morphology to category 4X lesions, but provides no specific recommendations regarding this entity (42). Despite their peculiar and unusual morphology on imaging, these lesions are staged according to the current TNM-staging 8th edition. CT-measurement on lesions (in particular Type IV according to Mascalchi or multilocular type according to Fintelmann) is—more than in other lung cancers—prone to variability. The cystic or non-solid component makes volumetric measurement challenging, and exophytic or endophytic components are often relatively small. In case of Mascalchi Type I and II lesions, measuring the total tumor size may overestimate the total tumor burden, since the largest part of the tumor contains air. Analogous to the new TNM classification for subsolid nodules which takes only in account the solid or invasive component for staging (43), one might consider a similar ‘adapted’ staging system for cystic airspace tumors, taking only the real tumorpart into account and not the entire lesion (including the—sometimes large—cystic airspace). Further research in this field is needed. While the large cystic component is clearly visible on CT, this may not always be the case on resection specimens. Since cystic lesions can be small and changes subtle, volumetric CT imaging with careful evaluation of the cystic airspace wall on thin slices, is advised. It remains unclear to what extent low-dose imaging may alter the detection of subtle findings and minimal changes in evolution.
Conclusions
Knowledge of the uncommon lung cancer manifestation, ‘lung cancer associated with cystic airspaces’, by all specialties involved in pulmonology and thoracic oncology is mandatory for recognition and early diagnosis. With the increased use of CT and emerging role of lung cancer screening, it is not unlikely that these tumors will be more frequently encountered in the future. Special attention should be made on CT to focal or diffuse wall thickening and consolidation or ground glass abutting or interspersed with cystic airspaces. New cystic lesions also merit attention and close surveillance is warranted in these cases.
A clear definition and uniform nomenclature for this specific lung cancer entity will be essential for future studies, since currently this group of lesions is heterogeneous, both on imaging and histopathology. Although the rarity of lung cancer associated with cystic airspaces will hamper larger studies, more data is needed to confirm current knowledge, to increase understanding of pathogenesis, to develop specific guidelines for follow-up, to possibly adapt the staging system and to give more insight in survival. In this respect, future lung cancer screening might fulfill part of this role by recognition and dedicated registration of this uncommon manifestation of primary lung cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Womack NA, Graham EA. Epithelial metaplasia in congenital cystic disease of the lung: its possible relation to carcinoma of the bronchus. Am J Pathol 1941;17:645-54.5.
- Anderson HJ, Pierce JW. Carcinoma of the bronchus presenting as thin-walled cysts. Thorax 1954;9:100-5. [Crossref] [PubMed]
- Peabody JW, Rupnik EJ, Hanner JM. Bronchial carcinoma masquerading as a thin-walled cyst. Am J Roentgenol Radium Ther Nucl Med 1957;77:1051-4. [PubMed]
- Goldstein MJ, Snider GL, Liberson M, et al. Bronchogenic carcinoma and giant bullous disease. Am Rev Respir Dis 1968;97:1062-70. [PubMed]
- Prichard MG, Brown PJ, Sterrett GF. Bronchioloalveolar carcinoma arising in longstanding lung cysts. Thorax 1984;39:545-9. [Crossref] [PubMed]
- Aronberg DJ, Sagel SS, LeFrak S, et al. Lung carcinoma associated with bullous lung disease in young men. AJR Am J Roentgenol 1980;134:249-52. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Wang X, Tao YX, Zhang M, et al. Solitary thin-walled cystic lung cancer with extensive extrapulmonary metastasis: a case report and review of the literature. Medicine (Baltimore) 2018;97:e12950. [Crossref] [PubMed]
- Sheard S, Moser J, Sayer C, et al. Lung cancers associated with cystic airspaces: underrecognized features of early disease. RadioGraphics 2018;38:704-17. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Truong MT, Ko JP, Rossi SE, et al. Update in the evaluation of the solitary pulmonary nodule. RadioGraphics 2014;34:1658-79. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Snoeckx A, Reyntiens P, Desbuquoit D, et al. Evaluation of the solitary pulmonary nodule: size matters, but do not ignore the power of morphology. Insights Imaging 2018;9:73-86. [Crossref] [PubMed]
- Walter JE, Heuvelmans MA, Oudkerk M. Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl Lung Cancer Res 2017;6:42-51. [Crossref] [PubMed]
- Rampinelli C, Calloni SF, Minotti M, et al. Spectrum of early lung cancer presentation in low-dose screening CT: a pictorial review. Insights Imaging 2016;7:449-59. [Crossref] [PubMed]
- Snoeckx A, Dendooven A, Carp L, et al. Wolf in sheep's clothing: primary lung cancer mimicking benign entities. Lung Cancer 2017;112:109-17. [Crossref] [PubMed]
- Kaneda M, Tarukawa T, Watanabe F, et al. Clinical features of primary lung cancer adjoining pulmonary bulla. Interact Cardiovasc Thorac Surg 2010;10:940-4. [Crossref] [PubMed]
- Fintelmann FJ, Brinkmann JK, Jeck WR, et al. Lung Cancers Associated with cystic airspaces: natural history, pathologic correlation, and mutational analysis. J Thorac Imaging 2017;32:176-88. [Crossref] [PubMed]
- Guo J, Liang C, Sun Y, et al. Lung cancer presenting as thin-walled cysts: An analysis of 15 cases and review of literature. Asia Pac J Clin Oncol 2016;12:e105-12. [Crossref] [PubMed]
- Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr 2015;39:102-8. [Crossref] [PubMed]
- Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Korol E. The correlation of carcinoma and congenital cystic emphysema of the lungs; report of ten cases. Dis Chest 1953;23:403-11. [Crossref] [PubMed]
- Lantuejoul S, Nicholson AG, Sartori G, et al. Mucinous cells in type 1 pulmonary congenital cystic adenomatoid malformation as mucinous bronchioloalveolar carcinoma precursors. Am J Surg Pathol 2007;31:961-9. [Crossref] [PubMed]
- Toyokawa G, Takada K, Okamoto T, et al. High Frequency of Programmed Death-ligand 1 Expression in Emphysematous Bullae-associated Lung Adenocarcinomas. Clin Lung Cancer 2017;18:504-511.e1. [Crossref] [PubMed]
- Maki D, Takahashi M, Murata K, et al. Computed tomography appearances of bronchogenic carcinoma associated with bullous lung disease. J Comput Assist Tomogr 2006;30:447-52. [Crossref] [PubMed]
- Son BY, Cho S, Yum SW, et al. The maximum standardized uptake value of preoperative positron emission tomography/computed tomography in lung adenocarcinoma with a ground-glass opacity component of less than 30 mm. J Surg Oncol 2018;117:451-6. [Crossref] [PubMed]
- Nakamura H, Saji H, Shinmyo T, et al. Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake in positron emission tomography. Lung Cancer 2015;87:28-33. [Crossref] [PubMed]
- Erasmus JJ, Macapinlac HA. Low-sensitivity FDG-PET studies: less common lung neoplasms. Semin Nucl Med 2012;42:255-60. [Crossref] [PubMed]
- Araki T, Nishino M, Gao W, et al. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax 2015;70:1156-62. [Crossref] [PubMed]
- Cosgrove GP, Frankel SK, Brown KK. Challenges in pulmonary fibrosis. 3: cystic lung disease. Thorax 2007;62:820-9. [Crossref] [PubMed]
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc 2003;78:744-52. [Crossref] [PubMed]
- Sharma P, Mukherjee A, Karunanithi S, et al. Potential role of 18F-FDG PET/CT in patients with fungal infections. AJR Am J Roentgenol 2014;203:180-9. [Crossref] [PubMed]
- Sathekge M, Maes A, Van de Wiele C. FDG-PET imaging in HIV infection and tuberculosis. Semin Nucl Med 2013;43:349-66. [Crossref] [PubMed]
- Henschke CI, Yip R, Boffetta P, et al. CT screening for lung cancer: importance of emphysema for never smokers and smokers. Lung Cancer 2015;88:42-7. [Crossref] [PubMed]
- Naccache JM, Gibiot Q, Monnet I, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis 2018;10:3829-44. [Crossref] [PubMed]
- Oh SY, Kim MY, Kim JE, et al. Evolving early lung cancers detected during follow-up of idiopathic interstitial pneumonia: serial CT features. AJR Am J Roentgenol 2015;204:1190-6. [Crossref] [PubMed]
- Lee T, Park JY, Lee HY, et al. Lung cancer in patients with idiopathic pulmonary fibrosis: clinical characteristics and impact on survival. Respir Med 2014;108:1549-55. [Crossref] [PubMed]
- Scholten ET, Horeweg N, de Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol 2015;25:81-8. [Crossref] [PubMed]
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-54. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads
- Travis WD, Asamura H, Bankier AA, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2016;11:1204-23.


