Five years’ experience with a peripheral veno-arterial ECMO for mechanical bridge to heart transplantation
Introduction
Orthotopic heart transplantation (OHTx) remains the gold standard for the therapy of patients with advanced heart failure (HF), having a 10-year survival rate of 50% and a satisfactory quality of post-transplant life (1). However, in conditions of increased demand for donor hearts, OHTx is available only for small and strictly selected patient pool with advanced HF (2,3). In the case of donor heart shortage and an expanding the pool of patients waiting for OHTx, it is necessary to apply the alternative approach to decrease the mortality rate in heart transplant waiting list (4). Implantable long-term left ventricular assist device (LVAD) is the leading method of MCS not only for heart transplant candidates but also patients that are ineligible for OHT (destination therapy) (5,6). More than 40% of heart transplantation has been performed in patients with LVAD according to ISHLT registry data (7). However, in some clinical situations, it is impossible for LVAD to significantly improve hemodynamics such as biventricular CHF (8). LVADs is associated with a risk of thromboembolic, hemorrhagic, infectious, and other complications (9). The high acquisition cost of the device and post-implantation management are also limiting factors due to economic considerations (10,11). In guaranteed availability of donor hearts short-term (temporary) MCS may be an alternative approach for heart transplant candidates who need an urgent OHTx procedure (12,13). One of the most frequently used methods of temporary MSC before heart transplantation is veno-arterial extracorporeal membrane oxygenation (VA ECMO) (13,14). In the last few years, heart transplant team of Shumakov National Medical Research Center of Transplantology and Artificial Organs (Moscow, Russian Federation) began to apply peripheral VA ECMO (pVA ECMO) as the leading method of pretransplant short-term MSC.
The goal of study was to estimate results of using pVA ECMO as a method of short-term MCS in heart transplant candidates requiring urgent HT.
Methods
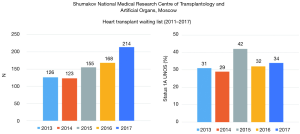
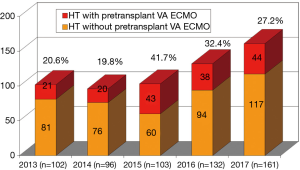
This study included 182 heart transplant candidates (160 (87.9%) men and 22 (12.1%) female, age from 12 to 76 (43±1.2) years) treated with a peripheral VA ECMO in our center in the period from 01. 01 .2013 to 31. 12. 2017 or 23.2% from all (n=786) patients included in our heart transplant waiting list from analyzed period (Figure 1).
Etiology of the advanced CHF was dilated cardiomyopathy [n=119 (65.4%)], coronary artery disease [n=46 (25.3%)], chronic cardiac allograft dysfunction [n=7 (3.8%)], congenital or acquired valve diseases [n=5 (2.7%)], peripartum cardiomyopathy [n=3 (1.6%)], hypertrophic cardiomyopathy [n=1 (0.5%)], restrictive cardiomyopathy [n=1 (0.5%)].
Sixteen patients (8.8%) underwent surgery in past: coronary artery grafting with/without LV reconstruction or with/without mitral valve repair [n=4 (2.2%)], heart valve repair [n=5 (2.7%)], and primary OHTx [n=7 (3.8%)].
Other comorbidities were hypertension [n=33 (18.1%)], chronic obstructive pulmonary disease [n=16 (8.8%)], non-hemodialysis-dependent chronic renal disease with estimated glomerular filtration rate (GFR) ≤40 mL/min/1.73 m2 [n=7 (3.8%)], carotid disease [n=12 (6.6%)], diabetes mellitus [n=4 (2.2%)], gastric or duodenum ulcer [n=6 (3.3%)], stroke [n=5 (2.7%)], pulmonary thromboembolism [n=4 (2.2%)], hepatitis B/C [n=2 (1.1%)], Dreifuss muscle dystrophy (c.de1619C mutation in EMD exon 6) [n=1 (0.5%)].
Transpulmonary gradient (TPG) and pulmonary vascular resistance (PVR), respectively, were 4–20 (11.2±2.5) mmHg and 1.9–5.6 (3.54±1.62) Wood’s Units. Thirty-four (18.7%) heart transplant candidates had TPG ≥15 mmHg and PVR ≥4 Wood’s Unit.
Seven (3.8%) patients were under mechanical ventilation, 6 (3.3%) noninvasive ventilator support, 4 (2.2%) intra-aortic balloon pump (IABP).
The indication for VA ECMO was rapidly progressing congestive heart failure (CHF) of Class 1 or 2 by the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) scale or cardiac arrest with the need of cardio-pulmonary resuscitation (CPR).
Open (surgical) or transcutaneous technique was used for installation of ECMO-cannulae in femoral vessels: arterial cannula (15–17 F) and venous cannula (21–28 F). In all cases, to prevent leg ischemia catheterization (single-lumen catheter 14 G) or cannulation (arterial cannula 8 or 10 F) was performed on the side of the femoral artery cannulation (Figure 2).

Continuous infusion of unfractionated heparin was used for anticoagulation during pVA ECMO. The activated clotting time (ACT) was maintained at a level of 130–150 s.
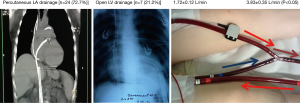
In cases of left ventricle (LV) distention and pulmonary edema, percutaneous transfemoral transseptal cannulation of the left atrium (LA) by additional venous ECMO-cannula (15–17 F) or direct left ventricle cannulation by additional single-lumen venous CPB-cannula (28–30 F) via left thoracotomy was used for unloading of left heart (Figure 3).
Statistical analysis
Continuous variables are presented as the means ± standard deviations for continuous variables and percentages for the qualitative variables. An unpaired t-test was used for normally distributed data, after assessment of the equality of the variances. All P values were two-tailed. Categorical variables are reported as percentages and compared using the Chi-square test. Univariate analyses were performed using Chi-square and Fisher’s exact tests for categorical variables. Survival and event-free survival were calculated using the Kaplan-Meier method. Statistical significance was defined as P<0.05. Statistical analyses were performed with the Biostat statistical software and the IBM SPSS version 20.0 software.
Results
In 100% (n=182) the peripheral cannulation technique via femoral vessels was used for installation of VA ECMO.
125 (68.7%) had clinical and hemodynamic indication for temporary MCS via VA ECMO corresponding to INTERMACS class 1, whereas 52 (28.6%) were in INTERMACS class 2. In several individual cases the indication for VA ECMO was extracorporeal CPR (ECPR) accounting for in 5 (2.7%) patients with in-hospital cardiac arrest. In these cases, cannulation was performed during manual (n=1) or mechanical (AutoPulse system) (n=4) chest compressions (Figure 4).
Surgical and percutaneous techniques of femoral cannulation were used in 29 (15.9%) and 153 (84.1%) patients, respectively. Femoral vessels of a single leg or both legs were used for cannulation in 120 (65.9%) and 62 (34.1%) patients, respectively.
Most patients [n=153 (84.1%)] were extubated within 1 hour after commencement of VA ECMO therapy. Twenty-nine pts (15.9%) were mechanically ventilated for more than 12 h after the initialization of VA ECMO. Four (2.2%) pts were later percutaneously tracheostomized for long-time invasive mechanical ventilation. Thirty-one (17.0%) patients were reintubated due to lung edema developed as a consequence of left heart overdistention (see below).
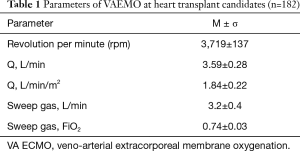
During VA ECMO, the extracorporeal blood flow was 2.2 to 4.5 (3.59±0.28) L/min or 1.84±0.22 L/min/m2 (Table 1). Inotropes were used in 100% of cases to maintain the residual heart pump function.
Full table
Twenty-nine (15.9%) patients required continuous venovenous hemofiltration (CVVH) for correction of hypervolemia or hyperhydration (anasarca), metabolic, electrolyte, and multiple organ dysfunction.
Despite the additional target therapeutic options (inotropic, diuretics, CVVH and noninvasive mechanical ventilation) 31 (17.0%) patients demonstrated lung edema (“white” lungs) due to LV overdistention and needed mechanical left heart volume decompression. Lung edema developed in 3.1±1.1 days after commencement of VA ECMO therapy. Percutaneous transfemoral cannulation of the LA (n=24) and LV drainage (n=7) were used for left heart decompression (Figure 2). LA and LV drainage was 1.72±0.12 and 3.60±0.38 L/min, respectively. LV drainage provided a more significant reduction of PCWP in comparison with LA drainage: from 35±5 to 13±6 mmHg versus from 29±3 to 17±3 mmHg (t=2.438, P=0.024). However, 4 (57.1%) from 7 patients with LV drainage were re-operated on had to be reopened due to significant postoperative blood loss (1,312±161 mL).
During VA ECMO, 16 (8.8%) of the 182 patients died. 3 (18.8%) patients with preexisting (before VA ECMO) massive LV thrombosis died from brain death after an acute thromboembolic cerebrovascular event. In most patients [n=13 (81.3%)] multiorgan failure and sepsis were the leading cause of death. Those patients (n=13) had more severe pre-MCS clinical status (Table 2).
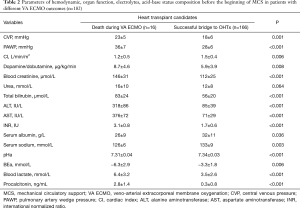
Full table
Significant (P<0.05) pre-MCS risk factors for the lethal outcome of heart transplant candidates supported by pVA ECMO were: creatinine ≥140 µmol/L, blood urea ≥15 mmol/L, total bilirubin ≥120 µmol/L, ALT ≥300 U/L, AST ≥300 U/L, INR ≥3.0, procalcitonin ≥3.0 ng/mL, and preexisting left ventricle thrombosis complicated by thromboembolic stroke with brain death following VA ECMO. Also, statistically significant factors for the fatal outcome following VA ECMO procedure were: transthoracic left ventricle drainage for left heart decompression and free hemoglobin ≥300 mg% (Table 3).
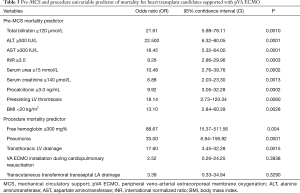
Full table
One hundred and sixty-six (91.2%) heart transplant candidates were successfully bridged to OHTx. The duration of VA ECMO before OHTx (n=166) ranged from 8 h to 40 (5.8±3.2) days. In 161 of those 166 patients, the length of VA ECMO was determined by the donor heart waiting time. In 5 patients, OHTx was delayed with the view to improving the pretransplant status and regression of multiorgan dysfunction.
Peri-operative period
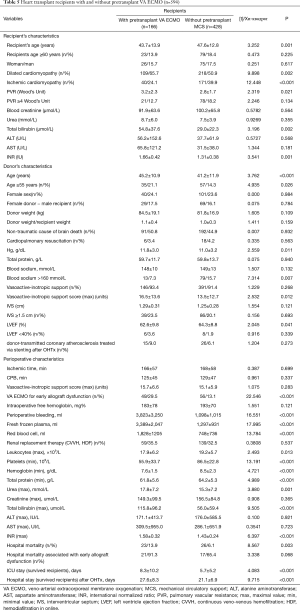
One hundred and sixty-six OHTs were performed in heart transplant recipients with pretransplant VA ECMO {27.9% from all OHT (n=594) in the analyzed period [2013–2017] (Figure 5)}, whereas 143 (86.1%) from 166 heart transplant recipients were discharged home. Twenty-three (13.9%) recipients died during the hospital period after OHT. Twenty-one (91.3%) from 23 recipients with pretransplant VA ECMO who died from multiple organ failure developed early cardiac allograft dysfunction. In this cohort of recipients pre-transplant levels of urea and total bilirubin were significantly higher (P<0.0001) (Table 4). Heart donors in the group of deceased recipients were also older (P=0.036), and more donors were of age 55 and older (21.1% vs. 14.3% (P=0.026). Also a perioperative period was associated with more blood loss and higher need for transfusion therapy with more severe renal and hepatic dysfunction, and higher need for renal replacement therapy.
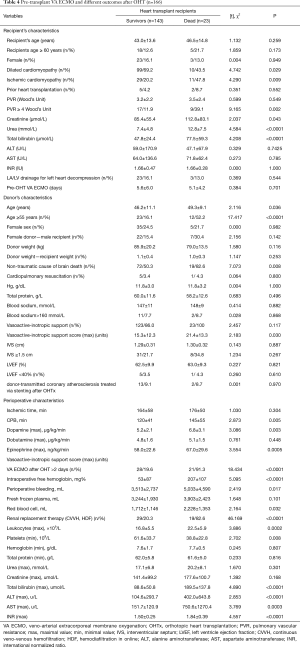
Full table
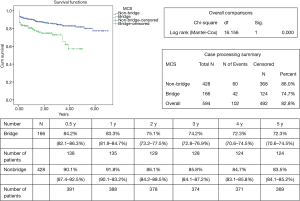
Comparison of heart transplant recipient cohorts with (n=166) and without (n=428) pretransplant VA ECMO demonstrated that recipients with pretransplant VA ECMO were younger (P=0.001), more frequently suffered from dilated cardiomyopathy (P=0.002), had higher preoperative levels of PVR (P=0.021), bilirubin (P=0.002) and INR (P=0.001) (Table 5). Cardiac donors in the cohort of pretransplant VA ECMO recipients were older (P=0.004), had significantly higher vasoactive-inotropic support (P=0.012) and lower LV ejection fraction (P=0.041). In the cohort of pre-transplant VA ECMO recipients perioperative period was characterized by the higher rate of early cardiac allograft dysfunction [91.3% vs. 65.4% (P=0.068)], higher blood loss and transfusion therapy. Hospital mortality was higher in the cohort with pre-transplant VA ECMO [13.9% vs. 6.1% (P=0.003)]. ICU and hospital stay among survived recipients was also longer (P<0.05) in the cohort with pre-transplant VA ECMO (Table 5, Figure 6). Nine significant factors predictive of hospital mortality were identified (Table 6). Early, mid-term and late results of OHTx in recipients bridged with VA ECMO were less promising (P<0.001) compared to recipients without pre-transplant MCS (Figure 6).
Full table

Full table
Discussion
According to the data from the ISHLT registry, approximately 50% of heart transplant recipients are treated with pretransplant MCS (7). Forty-two percent OHTx are performed after implantable LVADs. Taking into consideration potential risks and high costs of LVAD some heart transplant centers widely use methods of temporary MCS in heart transplant candidates requiring urgent OHTx (12,15). Results of OHTx in recipients with short-term pretransplant MCS are controversial, whereas some studies showed comparable early and long-term outcomes in recipients with pre-transplant temporal MCS (16,17).
In last time VA ECMO has been increasingly used for the treatment of critically ill patients with life-threatening pulmonary and cardiac disorders. One of the clinical applications of VA ECMO is MCS in heart transplant candidates (18). VA ECMO is a unique method of MCS that can be used in the same recipient before and after OHTx. It is suitable for urgent OHTx from donors with extended criteria and risk of early cardiac allograft dysfunction.
Transplant centers with expertise in urgent OHTx perform high numbers (10–38%) of OHTx in recipients with pretransplant VA ECMO (19,20). In our series, the annual amount of OHTx with pretransplant VA ECMO ranged from 19.8% to 41.7%. Such a high volume of OHTx in VA ECMO-supported patients was caused by an 8.6-fold increase in the number of patients on the waiting list and 6.7-fold increase in a proportion of patients requiring urgent OHTx (status 1A UNOS).
The duration of pretransplant VA ECMO can vary from several hours to several weeks, depending on the clinical status of the heart transplant candidate and availability of acceptable donor heart. The duration of VA ECMO treatment should not exceed 7–14 days. This duration of VA ECMO support may be sufficient to improve the pretransplant clinical status of patients without complications (bleeding, thromboembolism, infection, sepsis), that can have an unfavorable effect on the post-transplant outcomes or be fatal (21). However, patients with liver dysfunction and pulmonary complications (e.g., pneumonia) may demand more time for recovery and more extended MCS. In our study, preexisting liver dysfunction was a significant predictor of mortality for heart transplant candidates with VA ECMO (total bilirubin ≥120 µmol/L (21.61 OR, P=0.010), INR ≥3.0 UI (9.26 OR, P=0.0003).
The effectiveness of the pretransplant bridge with VA ECMO is variable. Chung et al. demonstrated that only 44% (31 out of 70) of patients were successfully bridged to OHTx (22). In a multicenter study by Barge-Caballero et al. 129 (76.3%) from 169 patients listed for urgent OHTx were successfully bridged by VA ECMO (13). In our study, the rate of the successful bridge to OHTx was 91.2% that may be explained by the high volume of VA ECMO procedures performed in our institution and center-specific management of patients with temporary MCS.
Our goal was to start VA ECMO before the development of severe multi-organ dysfunction, especially liver and renal dysfunction with their negative effects on pre-transplant MCS course and post-transplant survival. Preexisting liver dysfunction was shown to be a significant predictor for lethal outcomes in patients bridged to OHTx (23). Lechiancole et al. estimated that OHTx was associated with high early mortality in recipients bridged with VA ECMO and had high levels of multiorgan compromise (APACHE IV score ≥47 points) (20). In research, authors demonstrated that preexisting renal dysfunction was a significant predictor of post-transplant mortality for patients supported with VA ECMO (13,14,20,24). Cho et al. also demonstrated that post-transplant survival was low in recipients bridged with VA ECMO and patients had severe organ deteriorations [MELD UNOS score >24 (P=0.001), SOFA score >13 (P=0.068)] and duration of pre-transplant MCS was more than 5 days (P=0.056) (23).
Most studies on the use of VA ECMO as a bridge to transplantation have demonstrated poor early and long-term survival in heart recipients (13,14,24). We also found that early (hospital) and mid-term survival was worse than in recipients without pre-transplant MCS, however was comparable or even better than in other studies (Table 7).

Full table
In conclusion, VA ECMO is a useful tool of treatment of heart transplant candidates with life-threatening hemodynamic compromise (INTERMACS class 1 or 2). VA ECMO is a unique method of temporary MCS which may be extended for the early post-transplant period in the same recipient with early cardiac allograft dysfunction. It may be of high clinical significance particularly for urgent OHT from donors with extended criteria. However, early, mid-term and late results of OHTx in recipients bridged with VA ECMO are poorer than in recipients without pre-transplant MCS. Nevertheless, the volume of VA ECMO performed and centers expertise in VA ECMO management may be of paramount value significantly increasing survival of these demanding patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee of Shumakov National Medical Research Center of Transplantology and Artificial Organs (No. 21.12.12-1) and written informed consent was obtained from all patients.
References
- Ammar KA, Jaconson SJ, Maloney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007;115:1563-70. [Crossref] [PubMed]
- Buchholz S, Guentther SPW, Michel S, et al. Ventricular assist device therapy and heart transplantation: Benefits, drawbacks, and outlook. Herz 2018;43:406-14. [Crossref] [PubMed]
- Mehra MR, Canter CE, Hannan MM, et al. The 2016 International society for heart lung transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016;35:1-23. [Crossref] [PubMed]
- Garbade J, Barten MJ, Bittner HB, et al. Heart transplantation and left ventricular assist device therapy: two comparable options in end-stage heart failure? Clin Cardiol 2013;36:378-82. [Crossref] [PubMed]
- Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017;19:595-602. [Crossref] [PubMed]
- Puehler T, Ensminger S, Schoenbrodt M, et al. Mechanical circulatory support devices as destination therapy-current evidence. Ann Cardiothorac Surg 2014;3:513-24. [PubMed]
- Lund LH, Edward LD, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: thirty-second official adult heart transplantation; focus theme: early graft failure. J Heart Lung Transplant 2015;34:1244-54. [Crossref] [PubMed]
- Deschka H, Holthaus AJ, Sindermann JR, et al. Can Perioperative Right Ventricular Support Prevent Postoperative Right Heart Failure in Patients With Biventricular Dysfunction Undergoing Left Ventricular Assist Device Implantation? J Cardiothorac Vasc Anesth 2016;30:619-26. [Crossref] [PubMed]
- Robertson J, Long B, Koyfman A. The emergency management of ventricular assist devices. Am J Emerg Med 2016;34:1294-301. [Crossref] [PubMed]
- Tadmouri A, Blomkvist J, Landais C, et al. Cost-effectiveness of left ventricular assist devices for patients with end-stage heart failure: analysis of the French hospital discharge database. ESC Heart fail 2018;5:75-86. [Crossref] [PubMed]
- Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, et al. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients With Advanced Heart Failure. JACC Heart Fail 2017;5:110-9. [Crossref] [PubMed]
- Barth E, Durand M, Heylbroeck C, et al. Extracorporeal life support as a bridge to high-urgency heart transplantation. Clin Transplant 2012;26:484-8. [Crossref] [PubMed]
- Barge-Caballero E, Almenar-Bonet L, Gonzales-Vilchez F, et al. Clinical outcomes of temporary mechanical circulatory support as a direct bridge to heart transplantation: a nationwide Spanish registry. Eur J Heart Fail 2018;20:178-86. [Crossref] [PubMed]
- Jasseron C, Lebreton G, Cantrelle C, et al. Impact of heart transplantation on survival in patients on venoarterial membrane oxygenation at listing in France. Transplantation 2016;100:1979-87. [Crossref] [PubMed]
- Barge-Caballero E, Almenar-Bonet L, Villa-Arranz A, et al. Impact of short-term mechanical circulatory support with extracorporeal devices on postoperative outcomes after emergency heart transplantation: data from a multi-institution Spanish cohort. Int J Cardiol 2014;176:86-93. [Crossref] [PubMed]
- Jeevanandam V, Song T, Onsager D, et al. The first-in-human experience with a minimally invasive, ambulatory, counterpulsation heart assist system for advanced congestive heart failure. J Heart Lung Transplant 2018;37:1-6. [Crossref] [PubMed]
- Umakanthan R, Hoff SJ, Solenkova N, et al. Benefits of ambulatory axillary intra-aortic balloon pump for circulatory support as bridge to heart transplant. J Thorac Cardiovasc Surg 2012;143:1193-7. [Crossref] [PubMed]
- Sauer CM, Yuh DD, Bonde P. Extractorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015;61:31-6. [Crossref] [PubMed]
- D’Alessandro C, Golmard JL, Lebreton G, et al. High-urgency waiting list for cardiac recipients in France: single-centre 8-year experience. Eur J Cardiothorac Surg 2017;51:271-8. [PubMed]
- Lechiancole A, Sponga S, Isola M, et al. Heart transplantation in patients supported by ECMO: is the APCHE IV score a predictor of survival? Artif Organs 2018;42:670-3. [Crossref] [PubMed]
- Wang SS, Ko WJ, Chen YS, et al. Mechanical bridge with extracorporeal membrane oxygenation and ventricular assist device to heart transplantation. Artif Organs 2001;25:599-602. [Crossref] [PubMed]
- Chung JC, Tsai PR, Chou NK, et al. Extracorporeal membrane oxygenation bridge to adult heart transplantation. Clin Transplant 2010;24:375-80. [Crossref] [PubMed]
- Cho YH, Yang JH, Sung K, et al. Extracorporeal life support as a bridge to heart transplantation: importance of organ failure in recipients selection. ASAIO J 2015;61:139-43. [Crossref] [PubMed]
- Zalawadiya S, Fudim M, Bhat G, et al. Extracorporeal membrane oxygenation support and post-heart transplant outcomes among United States adults. J Heart Lung Transplant 2017;36:77-81. [Crossref] [PubMed]