
Impact of initial plasma presepsin level for clinical outcome in hospitalized patients with pneumonia
Introduction
Pneumonia is one of the major causes of death in the world. In Japan, pneumonia is the third leading cause of death after malignant neoplasms and heart diseases (1). To improve management of pneumonia, early identification of patients with poor prognosis is important. Therefore, many pneumonia severity assessment scales have been introduced worldwide. Pneumonia severity index (PSI) is a reliable severity assessment scale and recommended by Infectious Diseases Society of America and American Thoracic Society (2,3). In Japan, A-DROP score proposed by Japanese Respiratory Society is a common severity assessment scale for pneumonia. The A-DROP score is composed of 5 factors; age (≥70 years for men and ≥75 years for women); dehydration (≥21 mg/dL of blood urea nitrogen); respiratory failure (<90% of oxygen saturation); orientation disturbance; and low blood pressure (<90 mmHg of systolic blood pressure) (4).
In addition to these severity assessment scales, biomarkers such as procalcitonin and pro-adrenomedullin have been introduced for predicting prognosis of patients with pneumonia (5,6). Presepsin, a soluble N-terminal fragment of the cluster of differentiation (CD) marker protein 14, is known as an early diagnostic biomarker of sepsis. Previous studies in sepsis patients showed that high plasma presepsin level was associated with high mortality rate (7,8). However, the usefulness of presepsin for predicting clinical outcomes in pneumonia has not been evaluated enough. To our knowledge, only two previous pneumonia studies mentioned the relationship between plasma presepsin level and mortality (9,10). In this single-center retrospective study, we evaluated the prognostic value of plasma presepsin level in patients hospitalized due to pneumonia. Moreover, we also evaluated the relationship between plasma presepsin level and pneumonia etiology, and the correlation factors with plasma presepsin level.
Methods
Subjects
Patients hospitalized in Nagoya Tokushukai General Hospital (a 350-bed teaching hospital; Kasugai city, Aichi prefecture, Japan) due to pneumonia from May 2016 through November 2017 were reviewed using electronic medical records. The definition of pneumonia was presence of new radiographic infiltrates and exhibition of at least 2 compatible clinical symptoms (body temperature >37.5 °C, productive cough, chest pain, shortness of breath, and crackles on auscultation) (11). Patients under 18 years, hospitalized in the last 90 days, with hospital-acquired pneumonia, or transferred from other hospitals were excluded. During the study period, 231 pneumonia patients who satisfied both inclusion and exclusion criteria were hospitalized. Of these, 172 patients (74.5%) who underwent measurement of plasma presepsin level on admission were included in the study.
Study design
This study was aimed to investigate the following: (I) relationship between plasma presepsin level and 30-day mortality; (II) relationship between plasma presepsin level and pneumonia etiology; and (III) correlated factors with plasma presepsin level.
The following variables were retrieved from electronic medical records: (I) demographic characteristics (age, sex, code status, and site of residence); (II) coexisting illnesses; (III) clinical condition on hospital admission (body temperature, blood pressure, mental status, percutaneous oxygen saturation, and heart rate); (IV) laboratory and radiographic findings on hospital admission; (V) causative pathogens; (VI) regimens of intravenous antibiotics; and (VII) in-hospital mortality within 30 days of admission.
The A-DROP score was calculated based on retrieved information (4). The definition of disease severity drawn from A-DROP score was the following: 0, mild; 1 or 2, moderate; 3, severe; 4 or 5, super-severe (4). The PSI class was categorized using the prediction rule proposed by Fine et al. (2). The PSI class was stratified from 1 to 5, and the predicted 30-day mortality increased in accordance with the class (2). A do-not-resuscitate order was defined if such an order was mentioned in electronic medical records. Causative pathogens were diagnosed as per following criteria: (I) for Streptococcus pneumonia, 3+ growth of sputum culture, positive pleural fluid culture, or the presence of antigen in urine; (II) for Legionella pneumophila, the presence of antigen in urine; and (III) for other bacteria, 3+ growth of sputum culture or positive pleural fluid culture. The blood collection tube containing EDTA-2Na was employed for the measurement of plasma presepsin level. The measurement was performed immediately after blood sampling using a chemiluminescent enzyme immunoassay on a fully automated STACIA® immunoanalyzer (LSI Medience Corporation, Tokyo, Japan), and the result was derived within 30 minutes including centrifugation time. The normal reference value of plasma presepsin was less than 314 pg/mL. Other biochemical markers were assayed using standard methods. The study protocol was approved by the research ethics committee of Tokushukai group (approval number: TGE01003-016).
Statistical analyses
Data are expressed as numbers (%) or median (25th to 75th percentile range). Differences between the two groups were tested using the Mann-Whitney U test for continuous variables, and the Fisher’s exact test for categorical variables. Correlations between plasma presepsin level and other factors were assessed using the Spearman’s test. Interpretations of correlation coefficient (rs) were defined according to the following criteria: 0.90 to 1.00 (−0.90 to −1.00), very high correlation; 0.70 to 0.90 (−0.70 to −0.90), high correlation; 0.50 to 0.70 (−0.50 to −0.70), moderate correlation; 0.30 to 0.50 (−0.30 to −0.50), low correlation; and 0.00 to 0.30 (0.00 to −0.30), negligible correlation (12).
Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal cut-off value of plasma presepsin level for predicting 30-day mortality, and area under the curve with 95% confidence interval (CI) was calculated to evaluate the ability of each potential parameter for predicting 30-day mortality. Two survival curves differentiated with the optimal cut-off value of plasma presepsin level were plotted using the Kaplan-Meier survival analysis, and compared the two curves using the log-rank test. A two-tailed P value of <0.05 was considered statistically significant.
Statistical analyses for the Mann-Whitney U test, the Fisher’s exact test, the Spearman’s test, the Kaplan-Meier survival analysis, and the ROC curve analysis were performed using Ekuseru-Toukei 2012 (Social Survey Research Information Co., Ltd., Tokyo, Japan). The 95% CI of each ROC curve was drawn using ROC-KIT 2011 (Department of Radiology, The University of Chicago, IL, USA).
Results
Subjects’ characteristics
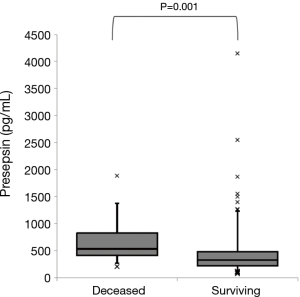
One hundred and seven patients (62.2%) were from the community, and 65 patients (37.8%) were from nursing homes. Seventeen patients (9.9%) died within 30 days of admission. Table 1 shows the comparison between deceased and surviving patients. The deceased patients had active malignancies (P=0.026) and more frequent do-not-resuscitate orders (P=0.017) compared with the surviving patients. Altered mental status (P<0.001) and hypoxia (P=0.004) were more frequently observed on admission in the deceased patients than in the surviving patients. The deceased patients had a smaller leukocyte count (P=0.049), hematocrit (P=0.028), and serum albumin level (P<0.001) compared with the surviving patients. Plasma presepsin levels were significantly higher in the deceased patients than in the surviving patients (539 vs. 334 pg/mL, P=0.001, Figure 1). The deceased patients had a higher A-DROP score (P<0.001) and more severe PSI class category (P<0.001) compared with the surviving patients. Concerning intravenous antibiotic regimens, anti-pseudomonal β-lactams were more frequently used in the deceased patients (P=0.008).
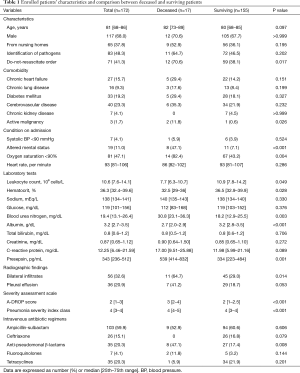
Full table
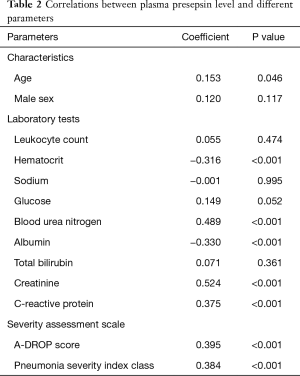
Correlated between plasma presepsin level and other factors
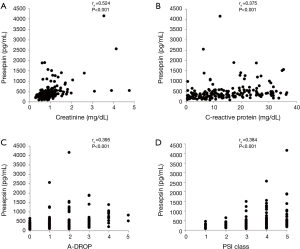
Table 2 presents the correlation between the plasma presepsin level and other factors. A moderate positive correlation was observed between the plasma presepsin level and serum creatinine level (rs =0.524, P<0.001, Figure 2A). Low positive correlations were observed between the plasma presepsin level and blood urea nitrogen (rs =0.489, P<0.001), serum C-reactive protein level (rs =0.375, P<0.001, Figure 2B), A-DROP score (rs =0.395, P<0.001, Figure 2C), and PSI class (rs =0.384, P<0.001, Figure 2D). The plasma presepsin level had low negative correlations with hematocrit (rs =−0.316, P<0.001) and serum albumin level (rs =−0.33, P<0.001).
Full table
Pneumonia etiology
Causative pathogens were identified in 83 patients (48.3%, Table 3). The most common pathogen was Streptococcus pneumoniae (22.7%). The plasma presepsin level did not differ between patients with and without identification of causative pathogens (378 vs. 312 pg/mL, P=0.112). Furthermore, the plasma presepsin level did not differ significantly between any two groups of causative pathogens.
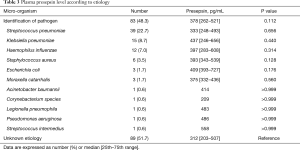
Full table
Prognostic value for 30-day mortality
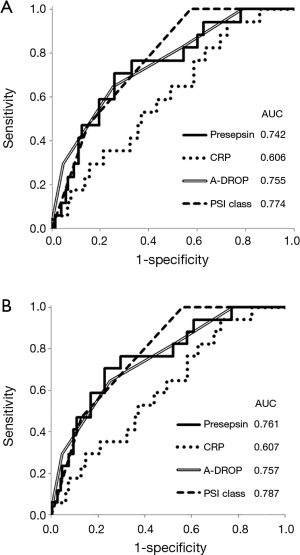
The areas under ROC curves for predicting 30-day mortality were 0.742 (95% CI: 0.621–0.863) for plasma presepsin level, 0.606 (95% CI: 0.476–0.736) for serum C-reactive protein level, 0.755 (95% CI: 0.634–0.877) for A-DROP score, and 0.774 (95% CI: 0.692–0.855) for PSI class (Figure 3A). Excluding seven patients with chronic kidney diseases, in remaining patients, the areas under ROC curves for predicting 30-day mortality were 0.761 (95% CI: 0.641–0.881) for plasma presepsin level, 0.607 (95% CI: 0.470–0.745) for serum C-reactive protein level, 0.757 (95% CI: 0.645–0.870) for A-DROP score, and 0.787 (95% CI: 0.708–0.867) for PSI class (Figure 3B).
Optimal cut-off value of plasma presepsin level for 30-day mortality
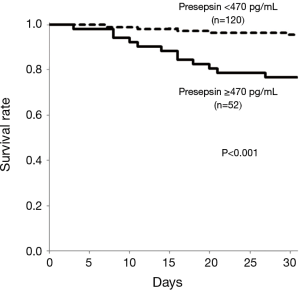
The optimal cut-off value derived from ROC curve analysis was 470 pg/mL of plasma presepsin level for predicting 30-day mortality. At the cut-off value, the sensitivity was 70.6%, the specificity was 74.2%, the positive predictive value (PPV) was 23.1%, and the negative predictive value (NPV) was 95.8% respectively. Using Kaplan-Meier survival analysis, patients with plasma presepsin level ≥470 pg/mL on admission (n=52) had significantly higher 30-day mortality than those with plasma presepsin level <470 pg/mL on admission (n=120) (P<0.001, Figure 4).
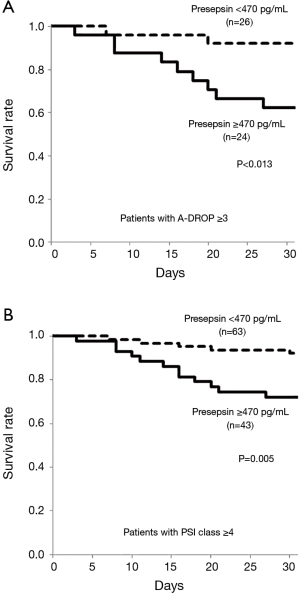
Among patients with A-DROP score ≥3, those with plasma presepsin level ≥470 mg on admission (n=24) had significantly higher 30-day mortality than those with plasma presepsin level <470 pg/mL (n=26) (P=0.013, Figure 5A). Similarly, among patients with PSI class ≥4, those with plasma presepsin level ≥470 mg on admission (n=43) had significantly higher 30-day mortality than those with plasma presepsin level <470 pg/mL (n=63) (P=0.005, Figure 5B).
Discussion
The present study showed that in patients hospitalized due to pneumonia, plasma presepsin level on admission (I) was significantly higher in deceased patients than in surviving patients, (II) was moderately but significantly correlated with patients’ kidney function, (III) was not associated with the etiology of pneumonia, and (IV) could be a useful predictor of 30-day mortality in pneumonia patients.
Presepsin has been recognized as an early diagnostic biomarker for sepsis because of its rapid response to systemic inflammation (13). However, limited information is available about clinical significance of presepsin in patients with pneumonia. The present study found that plasma presepsin level on hospital admission was significantly higher in deceased patients than in surviving patients. This finding is according with a previous study presented by Klouche et al., which mentioned that the optimal cut-off value of plasma presepsin for predicting 30-day mortality of pneumonia was 714 pg/mL (9). The value was higher than the one suggested through the present study (470 pg/mL). This discrepancy on the optimal cut-off plasma presepsin level might rise from the difference of study settings. The present study enrolled hospitalized pneumonia patients with various severities, while their study was performed on patients requiring intensive care. We also found that plasma presepsin level on admission had low but significant positive correlations with both A-DROP score and PSI class, which are widely used severity assessment scales for pneumonia. Similarly, Liu et al. reported that plasma presepsin level increased with the elevation of CURB-65 score, a pneumonia severity assessment scale proposed by British Thoracic Society (10,14).
PSI and A-DROP score are common severity assessment scales for pneumonia. However, some disadvantages were reported in these severity assessment scales. First, applying PSI in routine clinical practice may be difficult because its calculation is rather complex (15). Second, these severity assessment scales can underestimate the potential severity of pneumonia in young patients (16). Third, it is often difficult for clinicians to evaluate changes of mental status due to pneumonia in elderly patients (17). As compared to these severity assessment scales, the measurement of plasma presepsin level has an advantage because the definite value can be derived within 30 minutes of blood sampling. Among the patients with A-DROP score ≥3 or PSI class ≥4, significantly higher 30-day mortality was observed if they had plasma presepsin level ≥470 pg/mL on admission. The present study showed that the combination of plasma presepsin level and existing severity assessment scales was a potential useful method to detect pneumonia patients with high predicted mortality.
The present study showed that plasma presepsin level had a moderate but significant positive correlation with serum creatinine level. Previous reports suggested that kidney function has a large impact on plasma presepsin level (18,19). Nagata et al. reported that plasma presepsin level increased with a decrease in patients’ glomerular filtration rate (20). Furthermore, Nakamura et al. showed that plasma presepsin level was not a reliable diagnostic biomarker of sepsis in patients with severe kidney injury due to its high plasma level (21). The present study showed that plasma presepsin level on admission was a bit better for predicting 30-day mortality in patients without chronic kidney diseases. Thus, it is important to monitor patients’ kidney function while evaluating plasma presepsin level.
We found a low positive correlation between plasma presepsin and serum C-reactive protein levels. C-reactive protein is an acute-phase protein synthesized in hepatocytes responding to infection, inflammation, tissue damage, and malignant neoplasm (22). Presepsin is also an acute inflammatory protein synthesized in monocytes and macrophages during systemic bacterial infection (23). The present study cohort mainly comprised of patients with bacterial infection. This may explain the positive correlation between C-reactive protein and presepsin.
The hematocrit and serum albumin level showed low negative correlations with plasma presepsin level. Inflammation is known to be a cause of anemia because of suppressing erythropoiesis and shortening erythrocyte survival (24). An in-vitro study showed that interleukin-6, an inflammatory protein secreted by T lymphocytes and macrophages, directly impaired hemoglobin production and erythroid maturation (25). Hypo-albuminemia is also observed during inflammation due to its decreasing synthesis and increasing catabolism (26). Inflammation caused by pneumonia might be a major reason of the observed negative correlations.
The relationship between plasma presepsin levels and pathogens is not clear. Stoma et al. showed that plasma presepsin level was frequently elevated in patients with gram-negative bacteremia (27). Concerning pneumonia, Qi et al. showed that patients with gram-negative bacterial pneumonia had significantly higher value of plasma presepsin level than those with gram-positive bacterial pneumonia (28). Presepsin is a soluble form of CD14 subtype, and CD14 is known as a receptor to bind lipopolysaccharide which is a major component of the outer membrane of gram-negative bacteria (29). However, CD14 was also reported to bind with peptidoglycan which is abundant in the cell walls of gram-positive bacteria (30). In fact, Endo et al. reported that plasma presepsin levels did not differ between gram-positive and gram-negative bacterial infections in patients with sepsis (31). Through the present study, we found that plasma presepsin level was not associated with etiology of pneumonia.
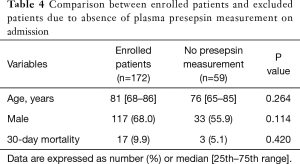
We must acknowledge some limitations of this study. First, the study included a small number of patients because it was a single-center study. To establish the clinical significance of plasma presepsin level in patients with pneumonia, a multi-center clinical study with a larger patient group is required. Second, the study cohort did not include outpatients. Therefore, our results may not be applicable to all patients with pneumonia. Third, 26% of hospitalized patients were excluded from the analysis due to the absence of plasma presepsin measurement on hospital admission. For the measurement of plasma presepsin level, an additional blood sample containing EDTA-2Na was required. This laborious blood sampling seemed to result in high absence rate of plasma presepsin measurement on admission in this study. There were no significant differences in age, sex distribution, and mortality between the included and excluded patients (Table 4). However, we cannot rule out the possibility that this high rate of exclusion might influence our results.
In conclusion, in this study, we showed that in patients hospitalized due to pneumonia, plasma presepsin level on admission was significantly higher in the deceased patients than in the surviving patients. Furthermore, our results suggest that in pneumonia patients, plasma presepsin level on admission could be a useful predictor of 30-day mortality and an additional prognostic biomarker on existing severity assessment scales.
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the research ethics committee of Tokushukai group (approval number: TGE01003-016).
References
- Japanese Ministry of Health, Labour and Welfare. Annual Health, Labour and Welfare Report 2017. Available online: . Accessed: 4th June 2018.http://www.mhlw.go.jp/english/wp/wp-hw11/dl/01e.pdf
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336:243-50. [Crossref] [PubMed]
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27-72. [Crossref] [PubMed]
- Miyashita N, Matsushima T, Oka M, et al. The JRS guidelines for the management of community-acquired pneumonia in adults: an update and new recommendations. Intern Med 2006;45:419-28. [Crossref] [PubMed]
- Schuetz P, Suter-Widmer I, Chaudri A, et al. Procalcitonin-Guided Antibiotic Therapy and Hospitalisation in Patients with Lower Respiratory Tract Infections (ProHOSP) Study Group. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J 2011;37:384-92. [Crossref] [PubMed]
- Bello S, Lasierra AB, Mincholé E, et al. Prognostic power of proadrenomedullin in community-acquired pneumonia is independent of aetiology. Eur Respir J 2012;39:1144-55. [Crossref] [PubMed]
- Ulla M, Pizzolato E, Lucchiari M, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care 2013;17:R168. [Crossref] [PubMed]
- Masson S, Caironi P, Fanizza C, et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: data from the multicenter, randomized ALBIOS trial. Intensive Care Med 2015;41:12-20. [Crossref] [PubMed]
- Klouche K, Cristol JP, Devin J, et al. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann Intensive Care 2016;6:59. [Crossref] [PubMed]
- Liu B, Yin Q, Chen YX, et al. Role of Presepsin (sCD14-ST) and the CURB65 scoring system in predicting severity and outcome of community-acquired pneumonia in an emergency department. Respir Med 2014;108:1204-13. [Crossref] [PubMed]
- Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-54. [Crossref] [PubMed]
- Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. 5th edition. Boston: Houghton Miffin; 2003.
- Wu J, Hu L, Zhang G, Wu F, et al. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS One 2015;10:e0133057. [Crossref] [PubMed]
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377-82. [Crossref] [PubMed]
- Chalmers JD, Hill AT. A powerful new severity score for community-acquired pneumonia but will anyone use it? Clin Infect Dis 2008;47:1363. [Crossref] [PubMed]
- Singanayagam A, Chalmers JD, Hill AT. Severity assessment in community-acquired pneumonia: a review. QJM 2009;102:379-88. [Crossref] [PubMed]
- Rudolph JL, Zanin NM, Jones RN, et al. Hospitalization in community-dwelling persons with Alzheimer's disease: frequency and causes. J Am Geriatr Soc 2010;58:1542-8. [Crossref] [PubMed]
- Chenevier-Gobeaux C, Trabattoni E, Roelens M, et al. Presepsin (sCD14-ST) in emergency department: the need for adapted threshold values? Clin Chim Acta 2014;427:34-6. [Crossref] [PubMed]
- Kotera A, Sagishima K, Tashiro T, et al. A validation of presepsin levels in kidney dysfunction patients: four case reports. J Intensive Care 2014;2:63. [Crossref] [PubMed]
- Nagata T, Yasuda Y, Ando M, et al. Clinical impact of kidney function on presepsin levels. PLoS One 2015;10:e0129159. [Crossref] [PubMed]
- Nakamura Y, Ishikura H, Nishida T, et al. Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesthesiol 2014;14:88. [Crossref] [PubMed]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805-12. [Crossref] [PubMed]
- Chenevier-Gobeaux C, Borderie D, Weiss N, et al. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta 2015;450:97-103. [Crossref] [PubMed]
- Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am 2014;28:671-81. [Crossref] [PubMed]
- McCranor BJ, Kim MJ, Cruz NM, et al. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis 2014;52:126-33. [Crossref] [PubMed]
- Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432-7. [Crossref] [PubMed]
- Stoma I, Karpov I, Uss A, et al. Diagnostic value of sepsis biomarkers in hematopoietic stem cell transplant recipients in a condition of high prevalence of gram-negative pathogens. Hematol Oncol Stem Cell Ther 2017;10:15-21. [Crossref] [PubMed]
- Qi ZJ, Yu H, Zhang J, et al. Presepsin as a novel diagnostic biomarker for differentiating active pulmonary tuberculosis from bacterial community acquired pneumonia. Clin Chim Acta 2018;478:152-56. [Crossref] [PubMed]
- Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431-3. [Crossref] [PubMed]
- Dziarski R, Tapping RI, Tobias PS. Binding of bacterial peptidoglycan to CD14. J Biol Chem 1998;273:8680-90. [Crossref] [PubMed]
- Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother 2012;18:891-7. [Crossref] [PubMed]


