Influence of comorbidities in long-term survival of chronic obstructive pulmonary disease patients
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide. Patients with COPD are prone to development of comorbidities, since they are usually at an advanced age, and their exposure to toxic gases and particles may affect all organs, and not just the lungs (1). Concomitant comorbidities can have an adverse effect on the overall health and survival of patients.
There are many comorbidities associated with COPD, such as hypertension, cardiovascular disease, diabetes and cancer (2), and the ratio of patients with at least one comorbidity is in the range of 70–97% (3-6). Although COPD is a complex disease with pulmonary and extra-pulmonary manifestations, the relationship between them is not yet fully understood. It is known, however, that age and smoking are common risk factors for COPD and many of the comorbid diseases that accompany it. It is also important to note that mortality in COPD with comorbidities is observed higher than the mortality in COPD alone in limited number of studies (7,8). Previous studies investigated the relationship between mortality and comorbidities, both individually or grouped, and there have been a few studies that identified a relationship between the number of comorbidities and long-term mortality.
In the present study, we investigated the prevalence of comorbidities diagnosed by a physician, and the relationship between the number of comorbidities and long-term mortality.
Methods
This study was conducted between January 1, 2012 and December 31, 2015, at the Sureyyapasa Research and Training Center for Chest Diseases and Thoracic Surgery (Istanbul, Turkey), which is affiliated with the Health Sciences University of the Ministry of Health. In this clinical study, patients hospitalized with a principal diagnosis of acute COPD exacerbation were included and their survival at 2 years were determined via the national death registry database. Between the aforementioned dates, we examined the files of all patients hospitalized with COPD exacerbation. The study protocol was approved by the hospital’s ethics committee in accordance with the Helsinki Declaration (date 12.03.2018, issue 11/038). No informed consent was obtained from the patients due to the retrospective recording of their data.
The patients
All patients had a diagnosis of COPD, and their diagnosis had been done by a pulmonary physician previously. A COPD diagnosis was based on a post-bronchodilator FEV1/FVC ratio less than 0.7, in the absence of a primary diagnosis of bronchiectasis, asthma, or any other significant respiratory disease. The patients also had symptoms and a history compatible with COPD (disease onset after 40 years of age, smoking history of at least 10 packs/year, or occupational exposure to irritant or toxic gases or biomass exposure). These patients were included in the study only once at their index hospitalization due to an exacerbation. The patients were hospitalized for one or more of the following indications: severely increased symptoms; new onset of cyanosis and peripheral oedema; confusion; lethargy; coma; use of accessory muscles for ventilation; significant comorbidities; failure to respond to initial treatment; judgment that treatment at home is insufficient; acidosis; persistent or worsening hypoxemia and/or severe or worsening hypercapnia and new onset arrhythmias.
Data collection
The files of all patients were examined, and data on age, gender, cigarette load (packs/year), body mass index (BMI), pulmonary function test values and the GOLD spirometric classification, duration of hospitalization, number of hospitalizations in the last year, duration of COPD, non-invasive mechanical ventilation (NIMV) use during hospitalization, transfer to the intensive care unit (ICU) and the physician-diagnosed comorbidities were recorded. Leukocyte, hematocrit, erythrocyte sedimentation rate, C-reactive protein (CRP), albumin and arterial blood gas (ABG) values were recorded, and chest X-rays were evaluated. The comorbidities of the patients were numerated separately, the number of comorbidities was determined, and the Charlson comorbidity indices were calculated. Based on this index, the score of 1, 2, 3 or 4 are given, respectively, ranking diseases from mild disease to serious to rate the severity of comorbid diseases (heart failure and myocardial infarction: 1, malignant diseases: 2, severe liver failure: 3, AIDS and metastatic solid tumors: 6 etc.). The Charlson comorbidity indices were then determined based on the total scores. The demographic findings and laboratory values of the patients with and without comorbidities were compared (Table 1). Survival for two years and dates of death were determined for all of the patients, and for those grouped based on the number of comorbidities (group 1: 0 comorbidity, group 2: 1 comorbidity, group 3: 2 comorbidities, group 4: 3 and more comorbidities). Two-year mortality was evaluated using national death registry database. This death registry database is updated continuously leaving no chance of delay in mortality reports and of missing and misleading data.
Full table
Statistical analysis
A descriptive analysis was used to investigate the patient demographics and clinical data, and the SPSS software package (SPSS for Windows, Version 20.0; SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Parametric values were represented as mean ± standard deviation. A student’s t-test was used for the evaluation of parametric distribution, while a Mann-Whitney U-test was used for non-parametric distribution. Categorical data (sex, gender, age, etc.) was analyzed with a Chi-square test for a paired analysis of the groups. Cox proportional hazards model was used to determine potential predictors of mortality. Age, cigarette load (pack/years), FEV1, arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), duration of the disease, BMI and the Charlson Comorbidity Index were selected as the independent variables for the Cox regression analysis. Regarding the comorbidity numbers, patients’ survival during two years after hospital discharge was evaluated by Kaplan-Meier survival analysis. The results were evaluated at a 95% CI, and a significance level at P<0.05 was accepted.
Results
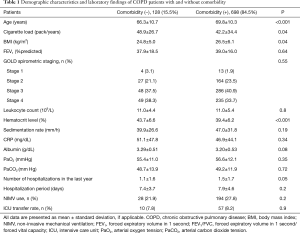
Of the 826 patients included in the present study, 639 (77.4%) were male and 187 (22.6%) were female, and the mean age was 69.3±10.4. The demographic data of the patients, laboratory and spirometric values, the number and duration of hospitalizations over the last year, NIMV use and the ratio of those referred to the ICU are presented in Table 1.
The mean age of the group with comorbidities was significantly higher than the group without comorbidity (69.8±10.3 versus 66.3±10.7; P<0.001). The hematocrit level was significantly higher in the group without comorbidities than in the comorbid group (43.7±6.6 versus 39.4±6.2; P<0.001). The mean value of BMI in the comorbid group was significantly higher than the other group (26.5±6.1 versus 24.8±5.0; P=0.04). History of hospitalization over the last year was higher in the group with comorbidities (1.5±1.7 versus 1.1±1.6), being very close to the level of statistical significance (P=0.05) (Table 1). Cigarette load (pack-year) was significantly higher in the group without comorbidities (48.9±26.7 versus 42.2±34.4; P=0.04) (Table 1). In contrast, there were no significant differences between the patients with and without comorbidity in terms of blood leukocyte, sedimentation, CRP, albumin, PO2, PaCO2 and FEV1 values and the distribution of the patients according to the GOLD spirometric classification. Likewise, it was found that the duration of hospitalization, the rate of NIMV and referral to the ICU rates were similar.
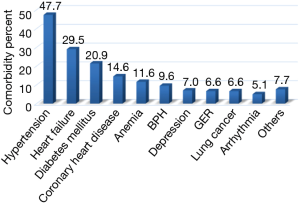
The most common comorbidities are shown in Figure 1. In terms of comorbidity frequency, 394 patients (47.7%) had hypertension, 244 (29.5%) had heart failure, 173 (20.9%) had diabetes mellitus (DM), 121 (14.6%) had coronary artery disease (CAD), 96 (11.6%) had anemia, 79 (9.6%) had benign prostatic hypertrophy (BPH), 58 (7.0%) had depression, 55 (6.6%) had gastroesophageal reflux (GER), 55 (6.6%) had lung cancer, 42 (5.1%) had arrhythmia and 64 (7.7%) had other comorbidities.
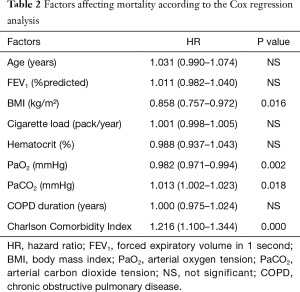
In the Cox regression analysis, a low BMI, low PaO2 value, high PaCO2 value and high Charlson comorbidity index were found to be related to mortality. The highest correlation was found with the Charlson comorbidity index, while no correlation was noted between age, cigarette load, FEV1, and duration of COPD and mortality (Table 2).
Full table
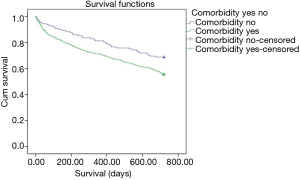
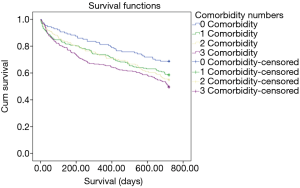
When the patients are classified in terms of comorbidity numbers, it was found that 128 patients (15.5%) had no comorbidities, 217 (26.3%) had one comorbidity, 229 (27.7%) had two comorbidities and 252 (30.5%) had three and more comorbidities. When the two-year mortality was analyzed with a Kaplan-Meier analysis, it was found that mortality in patients with comorbidities was significantly higher than those without comorbidities (HR: 1.44, P: 0.004) (Figure 2). When the long-term mortality was evaluated for the patients with one comorbidity, two comorbidities and three or more comorbidities, the mortality was found to be significantly higher with an increasing order compared to the patients without comorbidity (HR: 1.37, P: 0.032; HR: 1.40, P: 0.028; HR: 1.65, P: 0.000, respectively).The Kaplan Meier curves in Figure 3 is showing the statistically significant relationships between increasing number of comorbidities and mortality.
Discussion
This has been the first study to investigate the relationship between the number of comorbidities in COPD and long-term mortality. In the study, it was shown that the comorbidity rates of patients hospitalized for COPD exacerbation were high, and that comorbidities were the most significant factor affecting long-term mortality. In addition, as the number of comorbidities increased, mortality was found to increase significantly in the long-term follow-up.
Advanced age and exposure to toxic gases and dust, and especially cigarette smoke, are considered as the general risk factors for the development of higher rates of comorbidities in COPD patients. When the patients in the study were divided into two groups, being those with and without comorbidities, a significant difference was identified between the groups in terms of age, hematocrit, BMI, cigarette load and the number of hospitalizations over the last year. Analysis of these parameters revealed that age is an important factor for the occurrence of any comorbidity (66.3 versus 69.8, P<0.001). This is an expected outcome, considering the general increase in comorbidities with advancing age. Although in some of the previous studies, chronic comorbidities in COPD were found to be related to smoking (9,10), the other studies revealed that comorbidities were encountered more frequently in COPD patients regardless of smoking history (6,11). In the present study, patients without comorbidity had significantly higher cigarette loads than the others. This situation could be due to the fact that the patients with comorbidities stopped or lessened smoking to avoid further complications of the comorbidities at a higher rate, especially those with comorbidities of cardiac origin that appeared over time. When the hematocrit values of the patients with and without comorbidities were compared, the values were found to be significantly lower in patients with comorbidities (P<0.001). However, hematocrit values were not shown to affect mortality in our Cox regression model. In the present study, the number of hospitalizations due to COPD exacerbation was found higher in the comorbidity group (P=0.05). Similarly, Soler-Cataluña et al. found higher number of hospitalizations in COPD patients with higher comorbidity index (12), while Curkendall et al. demonstrated that cardiovascular comorbidities increased the risk of hospitalization (13). In the present study, BMI was higher in the group with comorbidity than in the other group (P=0.04), which can be explained with the fact that the patients with comorbidities are older, and therefore more immobile than those without comorbidities.
In the present study, 84.5% of the patients were found to have at least one comorbidity. Maleki-Yazdi et al. found the prevalence of comorbidity in COPD to be 95 percent, while Schnell et al. came up with a figure of 96.4% (4,14). von Manen et al., on the other hand, reported a comorbidity prevalence of 73% in their study group (15). Prevalence of comorbidity values may vary depending on the methodology of the studies. In our study, physician-diagnosed comorbidities were included in the study. The patients with complaints compatible with comorbidity, but who received no treatment and not been diagnosed previously, was not recorded as positive.
When the study patients with and without comorbidities were evaluated, 15.5 percent of all patients were found to have no comorbidity, 26.3% to have one comorbidity, 27.7% to have two comorbidities, and 30.5% to have three or more comorbidities. In their prospective studies involving 213 patients, Vanfleteren et al. recorded the prevalence of one comorbidity, two comorbidities and three or more comorbidities in 7.0%, 17.8% and 72.9% of the patients, respectively (16). In the ECLIPS cohort, the reported values for one comorbidity, two comorbidities and three or more comorbidities were 37%, 32% and 28%, respectively (6), while in the SUPPORT study, the rate of three and more comorbidities in COPD patients was identified as 39.9% (17). It can be understood that the results obtained in different studies may vary depending on the methodology.
In the present study, the mean number of comorbidities in all patients was found to be 1.86. On the other hand, the mean number of comorbidities only in COPD patients affected with comorbidities was found to be 2.2. In a study including 200 COPD patients and 200 controls, Mapel et al. identified a mean number of comorbidities of 3.7 (18), and in another study, the mean number of comorbidities was found to be 3.2 (4). Putcha et al. recorded a value of 3.3, while López Varela et al.’s finding was 1.2 (19,20). In general, the mean number of comorbidities that was identified falls between 1.2 and 4 (21). In the present study, physician-diagnosed diseases were considered as comorbidities, and that might have caused our rate to be slightly lower than the general rates.
In the present study, the five most common comorbidities in COPD were found to be HT (47.7%), heart failure (29.5%), DM (20.9%), ischemic heart disease (IHD) (14.6%) and anemia (11.6%), while in the study by Vanfleteren et al. (16), the most common comorbidities were DM (54%), IHD (53%) and HT (48%). Smith et al. (22) noted that hyperlipidemia, hypertension and anxiety were the most common comorbidities, while Miyazaki et al. found that HT (36%) to be the most prevalent, closely followed by GER (34%) and cardiac diseases (32%) (23). Stratev et al., on the other hand, found that the most common comorbidities were HT (70.4%), heart failure (47.4%), IHD (37.5%) and DM (21.4%) (24). In their study on 739 COPD patients, Koskela et al. found that HT (41%) was the most common comorbidity, followed by chronic psychiatric conditions (33%), heart disease (22%) and DM (15%) (3). Silver et al. found that HT (52.4%), IHD (31.7%) and DM (28.1%) were the most common comorbidities (25). In previous studies, although the prevalence and ranking of comorbidities changes, it can be seen that the three most common comorbidities were predominantly HT, cardiac diseases and DM, which is concordant with our study.
The Cox regression analysis applied in the present study showed that the most important parameters affecting mortality in the two years after index hospitalization, independent to the other variables, were a high Charlson Comorbidity Index (HR: 1.216) and low BMI (HR: 0.858). Other statistically significant parameters, such as low PaO2 (HR: 0.982) and high PaCO2 (HR: 1.013), have been shown to affect mortality, although their level of clinical significance is low. In their prospective study included patients with COPD exacerbations, Groenewegen et al. found that mortality rates one year after discharge were related to oral glucocorticoid use, high PaCO2 and age (26). Marti et al. found that advanced age, comorbidity, low BMI and Cor pulmonale were associated with overall mortality in COPD patients receiving long-term oxygen therapy. They further identified that mortality due to respiratory causes was related to the presence of comorbidities and low BMI (27). Almagro et al. claimed that low Katz index and FEV1, and high Charlson comorbidity index increased mortality three months after discharge, and suggested that the Charlson comorbidity index was an independent predictor of mortality (28). Chung et al. found that advanced age, low BMI and previous use of long-term oxygen therapy correlated with mortality rates five years after discharge (29). Yakar et al. showed that low PaO2, low BMI, low hematocrit levels and the Charlson comorbidity index increased the rates of mortality one year after discharge (30). In another study, a high Charlson Comorbidity Index was found to be the best predictor of mortality in deaths from all causes (31). Our study results were in many ways similar to those of the previous studies.
In the present study, patients with comorbidities were found to have a higher mortality than those without comorbidities two years after discharge from the hospital. As the number of comorbidities increased, mortality rates also increased. The highest clinical significance level was detected in patients with three or more comorbidities (P=0.000). In the ECLIPS study, it was found that patients with one, two, three and four or more comorbidities had higher mortality rates than those without comorbidities. In particular, the mortality rate of the patients with four or more comorbidities was found to be much higher than in the groups with fewer comorbidities (6). However, when calculating the number of comorbidities, the ECLIPS study also considered the comorbidities of non-COPD patients with normal lung function, in addition to those of patients with COPD. As a result, the relationship between the number of comorbidities and mortality in patients with COPD was not directly investigated. In a fairly large population study, Divo et al. identified a total of 79 comorbidities, and found that 12 of these, including various organ cancers, were associated with mortality (32). Also, when mortality after 52 weeks was investigated, the mean number of comorbidities was significantly higher in the COPD patients who had died. That study did not evaluate the relationship between the number of comorbidities and mortality. Punnam et al. showed that the hospital mortality of patients increased in parallel with the Deyo Modified Charlson Index score. Instead of the relationship between comorbidity number and mortality, they investigated the relationship between the comorbidity score and mortality (33). The literature has limited data on the relevant issue, and the studies investigating the effect of comorbidities on mortality in COPD generally examine the effects of diseases on mortality. Our study, on the other hand, can be considered as having a new approach that evaluates the relationship between the number of comorbidities and mortality in COPD patients.
To mention about the limitations, the apparent limitation of the present study was that we did not actively seek the comorbidities due to its retrospective design. However, our hospital’s electronic database system allowed us to see all other physician-diagnosed comorbid conditions of the patients correctly and to evaluate the treatments they receive for them. Since our database system is quite structured and provides detailed information about the past medical history of the patients, we do not think that missing comorbidities have significant impact on our results, if any.
Conclusions
The present study is a new one directly addressing the relationship between the long-term mortality in COPD and the number of comorbidities. We believe that our findings will contribute significantly to the limited literature in this field. In our study, comorbidities were found to be the most important parameter negatively influencing the mortality. The relevant data yielded that comorbidity rates in COPD patients with severe exacerbation were high, and the increasing number of comorbidities also increased the mortality rates in long-term follow-up. To conclude, our results strongly underline the fact again that, to be speaking about a better and more affective COPD management, the comorbidities must be actively sought and treated in COPD patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Hospital’s local ethics committee (date 12.03.2018, issue 11/038). No informed consent was obtained from the patients due to the retrospective recording of their data.
References
- Available online: , accessed September 30, 2018.http://www.goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- Hillas G, Perlikos F, Tsiligianni I, et al. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis 2015;10:95-109. [PubMed]
- Koskela J, Kilpelainen M, Kupiainen H, et al. Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med 2014;14:102. [Crossref] [PubMed]
- Maleki-Yazdi MR, Kelly SM, Lam SS, et al. The burden of illness patients with moderate to severe chronic obstructive pulmonary disease in Canada. Can Respir J 2012;19:319-24. [Crossref] [PubMed]
- Frei A, Muggensturm P, Putcha N, et al. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J Clin Epidemiol 2014;67:904-11. [Crossref] [PubMed]
- Miller J, Edwards LD, Agustí A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376-84. [Crossref] [PubMed]
- Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest 2005;128:2640-6. [Crossref] [PubMed]
- Terzano C, Conti V, Stefano F, et al. Comorbidity, hospitalization and mortality in COPD: Results from a Longitudinal Study. Lung 2010;188:321-9. [Crossref] [PubMed]
- Agustí AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:367-70. [Crossref] [PubMed]
- Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in the primary care. Chest 2005;128:2099-107. [Crossref] [PubMed]
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165-85. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martinez Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [Crossref] [PubMed]
- Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol 2006;16:63-70. [Crossref] [PubMed]
- Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med 2012;12:26. [Crossref] [PubMed]
- van Manen JG, Bindels PJ, Yzermans CJ, et al. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol 2001;54:287-93. [Crossref] [PubMed]
- Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728-35. [Crossref] [PubMed]
- Freeborne N, Lynn J, Desbiens NA. Insights about dying from the SUPPORT Project The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc 2000;48:S199-205. [Crossref] [PubMed]
- Mapel DW, Hurley JS, Frost FJ, et al. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med 2000;160:2653-8. [Crossref] [PubMed]
- Putcha N, Han MK, Martinez CH, et al. The comorbidities of COPD have a major impact on clinical outcomes, particularly in African Americans. Chronic Obstr Pulm Dis 2014;1:105-14. [Crossref] [PubMed]
- López Varela MV, Montes de Oca M, Halbert R, et al. Comorbidities and health status in individuals with and without COPD in five Latin American cities: the PLATINO study. Arch Bronconeumol 2013;49:468-74. [Crossref] [PubMed]
- Putcha N, Drummond MB, Wise RA, et al. Comorbidities and chronic obstructive pulmonary Disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med 2015;36:575-91. [Crossref] [PubMed]
- Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis 2014;9:871-88. [Crossref] [PubMed]
- Miyazaki M, Nakamura H, Chubachi S, et al. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res 2014;15:13. [Crossref] [PubMed]
- Stratev V, Petkova D, Dimitrova V, et al. Comorbidities of COPD in Bulgarian patients prevalence and association with severity and inflammation. Folia Med (Plovdiv) 2018;60:102-9. [Crossref] [PubMed]
- Silver H, Blanchette CM, Roberts M, et al. Prevalence of comorbidities in patients hospitalized for COPD exacerbations and impact on impatient mortality and hospital expenditures. Am J Respir Crit Care Med 2010;181:A5943.
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124:459-67. [Crossref] [PubMed]
- Marti S, Muñoz X, Rios J, et al. Body weight and comorbidity predict mortality in COPD patients treated with oxygen therapy. Eur Respir J 2006;27:689-96. [Crossref] [PubMed]
- Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD: the EPOC enservicios de medicina interna (ESMI) study. Chest 2012;142:1126-33. [Crossref] [PubMed]
- Chung LP, Winship P, Phung S, et al. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology 2010;15:1084-91. [Crossref] [PubMed]
- Yakar HI, Gunen H, Pehlivan E, et al. The role of tuberculosis in COPD. Int J Chron Obstruct Pulmon Dis 2017;12:323-9. [Crossref] [PubMed]
- Casanova C, Cote C, Torres JP, et al. Inspiratory to total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:591-7. [Crossref] [PubMed]
- Divo M, Cote C, Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155-61. [Crossref] [PubMed]
- Punnam SR. In-hospital mortality and long-term use of inhaled corticosteroids-reply. Arch Intern Med 2004;164:222; author reply 222-3. [Crossref] [PubMed]