
Difference in central nerve system metastasis during gefitinib or erlotinib therapy in patients with EGFR-mutated non-small cell lung cancer: a retrospective study
Introduction
Epidermal growth factor receptor (EGFR) mutations have been identified in a subset of patients with non-small cell lung cancer (NSCLC), and EGFR-tyrosine kinase inhibitors (TKIs) have been demonstrated to provide remarkable benefits in patients with NSCLC harboring EGFR-sensitive mutations. Despite the initial dramatic responses, most patients who receive EGFR-TKIs eventually acquire resistance to these drugs, and complete cure of patients with advanced EGFR-mutant NSCLC is rare (1,2). The incidence of central nervous system (CNS) metastasis, which includes brain metastasis and leptomeningeal metastasis, is higher among EGFR-mutant NSCLC patients than in NSCLC patients with wild-type EGFR (3). Furthermore, CNS metastasis considerably impairs the patients’ quality of life (QOL) and is a predictor of a poor outcome among patients with EGFR-mutant NSCLC (4). Therefore, prevention of CNS metastasis is an important treatment goal that would increase the beneficial effects of EGFR-TKIs in EGFR-mutant NSCLC patients. Some clinical studies have shown a possible difference in the incidence of CNS metastasis between patients treated with erlotinib and those treated with gefitinib (5-9). However, there is insufficient evidence about the preventive efficacy of these two drugs against CNS metastasis. Therefore, we planned a retrospective study to investigate the difference in the incidences of CNS metastasis between EGFR-mutant NSCLC patients receiving either of these two drugs as the first-line treatment in Japan.
Methods
Study population and data records
We enrolled EGFR-mutant NSCLC patients who had received gefitinib or erlotinib as the first-line EGFR-TKI treatment between January 2008 and December 2014 at the National Cancer Center Hospital Japan. All the patients were followed for the development of CNS lesions by computed tomography (CT) or magnetic resonance imaging (MRI). Patients who had uncommon EGFR mutations or who discontinued the EGFR-TKI treatments for any reason, and also patients who were not followed up for the development/progression of CNS lesions were excluded. We recorded the patients’ characteristics, including the age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS) before the start of the EGFR-TKI treatment, histological type of the primary lesion, history, clinical stage according to the 7th edition of Union for International Cancer Control (UICC), EGFR mutation subtype, history of radiation therapy (RT) for any CNS lesion(s) before the start of EGFR-TKI treatment, and the intervals between the brain imaging examinations. We also recorded the time of diagnosis or and of recurrence. after surgery for cancer, the dates of initiation and withdrawal of the EGFR-TKI treatments, the dates of last follow-up, the re-biopsy findings, and the patient outcomes from our institutional medical records. This study was conducted with the approval of the institutional review board of the National Cancer Center Hospital, Japan (No. 2015-038).
EGFR mutation analysis
EGFR mutations were evaluated in biopsy or surgical specimens or in specimens of pleural fluid. The detection of EGFR mutations was performed using a Scorpion amplification refractory mutation system (ARMS) or a high-resolution melting analysis (HRMA) (10) at the National Cancer Center Hospital, Japan.
Statistical analysis
Progression-free survival (PFS) was defined as the time from the date of initiation of EGFR-TKI treatment to the date of documentation of progressive disease (PD) or the date of death from any cause. Overall survival (OS) was defined as the time from the date of NSCLC diagnosis to the date of death from any cause. CNS PD was defined as the emergence of new CNS metastasis or progression of existing CNS metastasis. Time to CNS PD was defined as the time from the date of initiation of EGFR-TKI treatment to the date of diagnosis of CNS PD. Unidimensional measurements as defined by the Response Evaluation Criteria in Solids Tumors (RECIST), version 1.1, were used.
Fisher’s exact test was used to compare the incidences of CNS metastasis and the T790M mutation status between the patient groups treated with erlotinib and gefitinib. A log-rank analysis was used to compare the PFS, OS and time-to-CNS PD estimated by the Kaplan-Meier method. A two-sided P value of less than 0.05 was considered as denoting statistical significance. All of the statistical analyses were performed using SPSS ver. 23.0 (IBM, Armonk, NY, USA).
Results
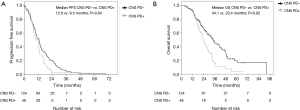
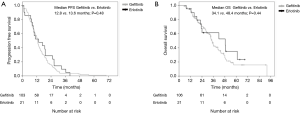
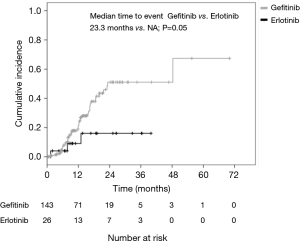
A total of 170 patients were enrolled in this study from January 2008 to December 2014. Overall, 144 patients had received gefitinib and 26 had received erlotinib treatment. The median ages of the patients in the gefitinib and erlotinib groups were 65.0 (range, 32–81) and 61.5 (range, 27–68) years, respectively. The histologic type of the cancer was adenocarcinoma in all of the cases. Exon 19 deletion/L858R point mutations were present in 82/62 (56.9%/43.1%) and 16/10 (61.5%/38.5%) patients in the gefitinib and erlotinib groups, respectively. Positive/negative statuses for CNS metastasis at the time of start of the EGFR-TKI treatment were observed in 38/106 (26.4%/73.6%) and 5/21 (19.2%/80.8%) patients in the gefitinib and erlotinib groups, respectively. The median interval until the first brain imaging examination was 3.0 (range, 1–8) months in the gefitinib group and 3.0 (range, 1–6) months in the erlotinib group (Table 1). CNS PD was detected in 29.9% of patients (43/144) in the gefitinib group and 11.5% of patients (3/26) in the erlotinib group. Thus, the incidence of CNS PD tended to be lower in the erlotinib group than that in the gefitinib group (11.5% vs. 29.9%; P=0.06) (Table 2). Additionally, none of the patients who had CNS PD in the erlotinib group progressed with isolated CNS metastasis, while 53.5% of patients (23/43) in the gefitinib group progressed with showed progression of an isolated CNS metastasis. In the patients with no existing CNS metastasis at the time of start of the EGFR-TKI treatment, the incidence of CNS PD metastasis was significantly lower in the erlotinib group (4.8%, 1/21 patients) than that in the gefitinib group (24.5%, 26/106 patients; P=0.04) (Table 2). The PFS and OS of patients without CNS PD were longer than those of patients with CNS PD (median PFS in patients without CNS PD vs. with CNS PD: 12.6 vs. 9.5 months; P=0.04, median OS in patients without CNS PD vs. with CNS PD: 34.1 vs. 23.4 months; P=0.02) (Figure 1). However, when the outcomes of the two treatment groups were compared, there was no significant differences in the PFS or OS between the gefitinib group and erlotinib group (median PFS in the gefitinib group vs. erlotinib group: 11.9 vs. 10.9 months; P=0.35; median OS in the gefitinib group vs. erlotinib group: 29.8 vs. 41.7 months; P=0.22) (Figure 2). In addition, among the patients with no existing CNS metastasis at the time of start of the EGFR-TKI treatment, no significant difference in the PFS or OS was noted between the two treatment groups (median PFS in the gefitinib group vs. erlotinib group: 12.8 vs. 13.6 months, P=0.48; median OS in the gefitinib group vs. erlotinib group: 34.1 vs. 48.4 months, P=0.44) (Figure 3). (We described opposite OS in gefitinib and erlotinib group in first manuscript). However, the time to CNS PD tended to be longer in the erlotinib group than that in the gefitinib group (Figure 4).

Full table

Full table

A re-biopsy was performed in 48 patients with failure of EGFR-TKI treatment. The incidence of EGFR T790M tended to be higher among the patients with CNS PD than among those without CNS PD, although the difference was not significant (66.7% in patients with CNS PD vs. 40.4% in patients without CNS PD; P=0.23). No significant differences were observed when the patients were compared according to the re-biopsy sites, EGFR-TKI used, and the EGFR mutation subtypes (Table 3).

Full table
Discussion
In this study, we showed that the frequency of CNS PD was lower in patients who received erlotinib treatment than in those who received gefitinib treatment. Furthermore, the difference was significant among patients with no existing CNS metastasis at the time of start of the EGFR-TKI treatments. The difference in the frequency of CNS PD between the erlotinib and gefitinib groups was consistent with the results of some previous clinical trials and retrospective studies. The reported frequency of CNS PD in patients treated with erlotinib is 2.9–8.0%, whereas that in the patients treated with gefitinib is 33–36.6% (5-9).
Li et al. reported the only direct comparative study between these two drugs. According to this study (11), the time-to-CNS PD was significantly longer in the erlotinib group than that in the gefitinib group (24 vs. 16 months, P=0.01), and the CNS PD rate at 18 months also tended to be lower in the erlotinib group than that in the gefitinib group (12.0% vs. 17.0%, P=0.181). Additionally, in the patients without preexisting brain metastasis at the time of start of the EGFR-TKI treatment, the median time-to-CNS PD tended to be longer in the erlotinib group than that in the gefitinib group (18 vs. 16 months, P=0.392). From these trials and the present results, it appears that erlotinib may be more effective for preventing the occurrence/progression of CNS metastasis than gefitinib in patients with EGFR mutations, especially in preventing the occurrence of CNS metastasis in those without existing CNS metastasis at the time of initiation of EGFR-TKI treatment.
The mechanism underlying the difference in the efficacy of the two drugs in preventing the occurrence/progression of CNS lesions remains unclear. However, based on the findings of several studies, we speculate that the difference may be caused by a higher penetration of erlotinib across the brain blood barrier. In early-phase trials, the recommend dose (RD) of erlotinib was 150 mg, which was equal to the maximum tolerated dose (MTD) (12). In contrast, the RD of gefitinib was 250 mg, which was only one-third of the MTD (13). Additionally, the concentration in the cerebrospinal fluid (CSF) of erlotinib and the CSF/plasma ratio of erlotinib were 34.7–186 nM and 2.5–13%, respectively, whereas, those of gefitinib were 8.2–13.87 nM and 1.1–1.3%, respectively (14-16). Furthermore, some case reports showed an improvement of the ECOG PS and neurologic symptoms in patients with brain metastasis or leptomeningeal metastasis after switching from first gefitinib treatment to erlotinib treatment (14,17). Our findings reflect these data and show the potential effectiveness of erlotinib for CNS metastasis.
Although erlotinib tended to prevent CNS metastasis, the PFS and OS showed no significant differences between the gefitinib and erlotinib treatment groups in the present study. This result could be attributable to the high rate of administration of next-line chemotherapy in both groups (gefitinib group: 69.4%; erlotinib group: 69.2%). In addition, the rate of RT, including stereotactic RT and whole-brain irradiation after the diagnosis of CNS PD was also high in both groups (gefitinib group: 76.7%; erlotinib group: 100%). RT for brain metastasis is a standard therapy for NSCLC patients with brain metastasis that has been shown to improve the CNS disease control rate, PS, and neurological symptoms (18,19). Therefore, the high rate of RT could have maintained or improved the patients’ PS and contributed to the administration of next-line chemotherapy. Recently, Magnuson et al. showed that RT followed by EGFR-TKI treatment yielded a longer OS than upfront EGFR-TKI treatment in EGFR-mutant NSCLC patients with brain metastasis (20). The present results and the retrospective studies suggest that RT followed by erlotinib treatment has the potential to improve the prognosis of patients with preexisting CNS metastasis.
We analyzed the incidence of the T790M mutation. Patients with CNS PD tended to have a higher incidence of T790M mutation than those without CNS PD, although the difference in the frequency was not significant. Osimertinib, a third-generation EGFR-TKI, has been demonstrated to show promising efficacy for NSCLC patients with the EGFR T790M mutation. Moreover, osimertinib was found to show favorable efficacy against CNS lesions; the CNS objective response rate was 70% and the median CNS PFS was 11.7 months (21).
The present study had several limitations. First, because it was a retrospective study performed at a single institution, some biases were inevitable. Erlotinib was granted approval after gefitinib in Japan, therefore, the possibility of a selection bias arising from the relatively small number of patients in the erlotinib group cannot be excluded. Second, CNS metastasis was detected using enhanced CT or MRI of the brain. The accuracy of CT imaging for the detection of brain metastasis may be inferior to that of brain MRI. Third, the number of patients with T790 mutation enrolled in the study was relatively small, and additional patients with this mutation need to be included to investigate the differences in the outcome according to the T790M status.
The incidence/progression of CNS metastasis tended to be low in the patients who received erlotinib treatment than in those who received gefitinib treatment. Additionally, the difference in the incidence was even more remarkable among patients who did not have CNS metastasis at the time of start of EGFR-TKI treatment. Our results suggest that erlotinib might be more effective for preventing CNS metastasis in patients with EGFR-mutant NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: S Kanda received research funding from AstraZeneca, Ono Pharmaceutical and AbbVie, and honoraria from AstraZeneca, Ono Pharmaceutical, Bristol-Myers Squibb, and Chugai. Y Goto has held consulting/advisory roles for Eli Lilly, Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Pfizer and Novartis, served on the speakers’ bureaus for AstraZeneca, Eli Lilly, Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, MSD, Shionogi Pharma and Novartis, and received research funding from AbbVie, Eli Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb and Ono Pharmaceutical. H Horinouchi has received research funding from Astellas, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Genomic Health, Merck Serono, MSD, Novartis, Ono Pharmaceutical and Taiho Pharmaceutical, and received honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Eli Lilly, Kyowa Hakko Kirin, MSD, Novartis, Ono Pharmaceutical and Taiho Pharmaceutical. Y Fujiwara has received research funding from AbbVie, AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Incyte, Merck Serono, MSD and Novartis, served on the speakers’ bureaus from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Novartis, Ono Pharmaceutical, Taiho Pharmaceutical and Sysmex, and held consulting/advisory roles for AstraZeneca, Bristol-Myers Squibb, Novartis and Ono Pharmaceutical. H Nokihara has received research finding form Merck Serono, Pfizer, Taiho Pharmaceutical, Eisai, Chugai, Eli Lilly, Novartis, Daiichi Sankyo, GlaxoSmithKline, Yakult, Quintiles, Astellas Pharma, AstraZeneca, Boehringer Ingelheim and Ono Pharmaceutical, and received honoraria from AstraZeneca, Ono Pharmaceutical, Eli Lilly, Bristol-Myers Squibb and Chugai. N Yamamoto has received research funding from Astellas, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Janssen pharmaceutical, Kyowa Hakko Kirin, Novartis, Ono Pharmaceutical, Pfizer, Quintiles, Taiho Pharmaceutical, and Takeda; and served on speakers' bureaus from AstraZeneca, Bristol-Myers Squibb, Chugai, Eli Lilly, Ono Pharmaceutical and Pfizer, and held consulting/advisory roles for Boehringer Ingelheim, Cimic, Eisai, OncoTherapy Science, Otsuka, and Takeda. Y Ohe has received research funding from AstraZeneca, Bristol-Myers Squibb, Chugai, Dainippon-Sumitomo, Eli Lilly, Ignyta, Kyorin, MSD, Novartis, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical and Takeda, and received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Eli Lilly, Kyorin, MSD, Novartis, Ono Pharmaceutical, Pfizer, Taiho Pharmaceutical and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was conducted with the approval of the institutional review board of the National Cancer Center Hospital Japan (No. 2015-038), in accordance with the principles laid down in the Declaration of Helsinki.
References
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Yamamoto N, Goto K, Nishio M, et al. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol 2017;22:70-8. [Crossref] [PubMed]
- Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005;103:2344-8. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Sugawara S, Oizumi S, Minato K, et al. Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol 2015;26:888-94. [Crossref] [PubMed]
- Park K, Yu CJ, Kim SW, et al. First-Line Erlotinib Therapy Until and Beyond Response Evaluation Criteria in Solid Tumors Progression in Asian Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer: The ASPIRATION Study. JAMA Oncol 2016;2:305-12. [Crossref] [PubMed]
- Lampson BL, Nishino M, Dahlberg SE, et al. Activity of erlotinib when dosed below the maximum tolerated dose for EGFR-mutant lung cancer: Implications for targeted therapy development. Cancer 2016;122:3456-63. [Crossref] [PubMed]
- Fukui T, Ohe Y, Tsuta K, et al. Prospective study of the accuracy of EGFR mutational analysis by high-resolution melting analysis in small samples obtained from patients with non-small cell lung cancer. Clin Cancer Res 2008;14:4751-7. [Crossref] [PubMed]
- Li MX, He H, Ruan ZH, et al. Central nervous system progression in advanced non-small cell lung cancer patients with EGFR mutations in response to first-line treatment with two EGFR-TKIs, gefitinib and erlotinib: a comparative study. BMC Cancer 2017;17:245. [Crossref] [PubMed]
- Yamamoto N, Horiike A, Fujisaka Y, et al. Phase I dose-finding and pharmacokinetic study of the oral epidermal growth factor receptor tyrosine kinase inhibitor Ro50-8231 (erlotinib) in Japanese patients with solid tumors. Cancer chemother Pharmacol 2008;61:489-96. [Crossref] [PubMed]
- Nakagawa K, Tamura T, Negoro S, et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 2003;14:922-30. [Crossref] [PubMed]
- Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol 2011;67:1465-9. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Zhao J, Chen M, Zhong W, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer 2013;14:188-93. [Crossref] [PubMed]
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415-9. [Crossref] [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]
- Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. [Crossref] [PubMed]


