
Classification and treatment of chronic obstructive pulmonary disease outpatients in China according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: comparison with GOLD 2014
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease characterized by persistent respiratory symptoms and airflow limitation (1). COPD is a leading cause of morbidity and mortality worldwide, with a global prevalence of 11.7% (2), and it is responsible for around 3 million deaths annually (3). The prevalence of COPD varies considerably among countries owing to differences in survey methods, diagnostic criteria, and analytical approaches (4-7). The incidence of COPD is gradually increasing over time. Recently, a large-scale epidemiological investigation in China showed that the overall prevalence of spirometry-defined COPD was 8.6%, accounting for 99.9 million adults (8). The prevalence was higher in people aged ≥40 years than in those aged 20–39 years.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines are widely used to guide clinical practice. The GOLD 2011 guidelines (9) combined symptomatic assessment with spirometric classification and/or risk of exacerbations. The revised GOLD 2014 guidelines (10) added history of hospitalization due to exacerbation in the preceding year as an approach to assessing exacerbation risk. However, increasing evidence suggests that the severity of airflow limitation is poorly related to the degree of breathlessness, health status, comorbidities, exercise capacity, and exacerbation risk (11,12). The degree of airflow limitation does not capture the heterogeneity of the disease (11). Additionally, previous studies have confirmed that a history of COPD exacerbation in the previous year is a strong predictor of future exacerbation (13,14). These findings highlight the potential limitations of too much focus on the forced expiratory volume in 1 s (FEV1) during prognostic and therapeutic decisions.
The GOLD 2017 guidelines (1) contain comprehensive revisions of the earlier guidelines. An important revision concerns the “ABCD” classification for the management of patients with COPD, which classifies patients into groups A (low risk, fewer symptoms), B (low risk, more symptoms), C (high risk, fewer symptoms), and D (high risk, more symptoms) (10). The GOLD 2017 classification criteria eliminated the degree of airflow limitation (based on spirometric grades) from the “ABCD” categorization system, requiring that patients be stratified into the four groups based only on symptoms by using either a dyspnea measure [the modified Medical Research Council (mMRC) dyspnea grade] or a health status measure [the COPD Assessment Test (CAT) score] in addition to COPD exacerbation history (1).
Only a few studies have reported on the utility of the new GOLD 2017 classification criteria in COPD populations. In Spain and the United States, a study of 819 COPD patients found that, compared to the GOLD 2015 criteria, the GOLD 2017 criteria reclassified a substantial proportion of patients from groups C–D to groups A–B (15). An analysis of COPD patients drawn from the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort also showed that GOLD 2017 reclassified a substantial proportion of GOLD 2011 patients from groups C–D to groups A–B (16). Similar results were reported in analyses of data from the Canadian Cohort of Obstructive Lung Disease (CANCOLD) and the Phenotypes of COPD in Central and Eastern Europe (POPE) cohorts (17,18). In addition, researchers reported strict adherence to the new GOLD 2017 guidelines likely reduced the treatment intensity (17), especially regarding the use of inhaled corticosteroids (ICS) (18,19).
In China, the impact of the GOLD 2017 revisions on patient categorization and subsequent treatment selection has been insufficiently studied. A national cross-sectional observational survey involving 11 medical centers in seven provinces in China concluded that GOLD 2017 shifted the overall distribution of patients to low-risk groups. Furthermore, a study showed that some of the characteristics of the new low-risk groups might in fact be associated with high risk of exacerbation (20). However, the impacts on treatment decisions remain unclear. Therefore, the aims of this study were as follows: (I) to evaluate how COPD patients in China, were reclassified by the 2017 GOLD classification criteria (compared to GOLD 2014); (II) to comprehensively characterize the four groups according to the GOLD 2014 and 2017 classification criteria; and (III) to describe the treatment of COPD patients and discuss the possible implications of the GOLD 2017 treatment recommendations on maintenance therapy for COPD.
Methods
Study design
The study was an observational, multicenter, cross-sectional study that recruited patients attending outpatient clinics at 12 representative tertiary hospitals in Hunan and Guangxi, China, from April 2016 to July 2018. All outpatients at these clinics who met the selection criteria were included. The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University, and written informed consent was obtained from all participants.
Population
Patients were included if they were aged ≥40 years and had been diagnosed with COPD according to the GOLD 2017 classification criteria (1). All included patients had dyspnea, chronic cough, chronic sputum production, and/or a history of exposure to risk factors, and persistent airflow limitation [defined spirometrically as postbronchodilator FEV1/forced vital capacity (FVC) <70%]. They had all been stable for a minimum of 4 weeks and had the ability to complete the CAT and mMRC questionnaires independently. All patients were required to have their history of exacerbations recorded in the previous 12 months (for categorization and quality control), which likely led to the inclusion of patients with more severe COPD.
Exclusion criteria were previous pulmonary or bronchial surgery, presence of other active or chronic respiratory diseases (such as asthma, bronchiectasis, severe sequelae of pulmonary tuberculosis, or lung cancer), and participation in any interventional clinical trials.
Study procedures
The following data was collected from patients: age, sex, height, weight, body mass index (BMI), education level, smoking history, FEV1% predicted, FEV1/FVC, number of exacerbations, number of hospitalizations due to exacerbation in the previous year, degree of baseline dyspnea (according to mMRC grade), symptoms (according to CAT score), and treatment protocols. These data were obtained using self-administered questionnaires, clinical records, and direct inquiry. Trained investigators independently extracted data from the questionnaires and clinical records, and trained doctors carried out data quality control. Consensus with the doctors was achieved for all data. As the study was a non-interventional observational study, each patient’s treatment protocol was selected by their physicians according to routine medical practice and local prescribing practice.
The most recent spirometric data available were documented. The severity of airflow limitation categories was defined according to the GOLD 2017 classification criteria: 1 (mild): FEV1 ≥80% predicted; 2 (moderate): FEV1 50–79% predicted; 3 (severe): FEV1 30–49% predicted; and 4 (very severe): FEV1 <30% predicted (1). An exacerbation was defined as an increase in or the new onset of more than one respiratory symptom (cough, sputum, sputum purulence, wheezing, or dyspnea) lasting ≥3 days and requiring treatment with an antibiotic or a systemic corticosteroid and/or hospitalization (21). Exacerbations separated by ≥14 days were considered distinct events (22). Evaluation of symptoms was based on the mMRC grades and CAT scores, which indicated whether the patient had fewer symptoms (mMRC grade 0–1 or CAT score <10) or more symptoms (mMRC grade ≥2 or CAT score ≥10) (1). When the two evaluations were inconsistent, the evaluation indicating more symptoms was used. Exacerbation risk was assessed based on airflow limitation in terms of the postbronchodilator FEV1% predicted (<50% or ≥50%), the number of COPD exacerbations in the previous year (≤1 or ≥2). Of note, having at least one hospitalization for a COPD exacerbation during the past year was considered to represent high risk. When the two evaluations were inconsistent, the evaluation indicating higher risk was used (9).
The GOLD 2014 classification criteria classified patients into the following groups: A (low risk, fewer symptoms), B (low risk, more symptoms), C (high risk, fewer symptoms), and D (high risk, more symptoms) (10). However, the GOLD 2017 classification criteria eliminated the degree of airflow limitation from the categorization system, and exacerbation risk was assessed only based on exacerbation history in the previous year, which stratified patients into low-risk (A–B) and high-risk (C–D) groups (1). The methods of assessing symptoms remained unchanged.
Statistical analysis
Data were entered into SPSS (version 22; IBM Corporation, Armonk, NY, USA) for analysis. Normally distributed continuous data were expressed as mean ± SD, non-normally distributed continuous data were expressed as medians (interquartile range), and categorical data were expressed as frequencies (percentage). Unordered categorical variables were compared using the chi-square or Fisher’s exact test, ordinal categorical variables were compared using the Mann-Whitney U or Kruskal-Wallis H test, and continuous variables were compared using the t-test (or the Mann-Whitney U test if the data were non-normal) or analysis of variance (ANOVA). Multivariate logistic regression analyses of the demographic variables (including age, BMI, education level, and pack-years of smoking) were used to identify independent risk factors for high risk of exacerbation (i.e., being in groups C–D). P<0.05 was considered statistically significant.
Results
Study population
From the original cohort of 4,481 patients in 12 medical centers, the following 3,203 patients were excluded: 129 patients were suffering from an exacerbation when recruited; 443 patients presented with other respiratory diseases; 1,640 patients had no mMRC grade; 808 patients had no pulmonary function test result; 99 patients had no records of exacerbation history; 9 patients were aged <40 years at enrollment; and 75 patients did not meet the COPD criteria. Data for the remaining 1,278 patients in Hunan were analyzed (Figure 1).
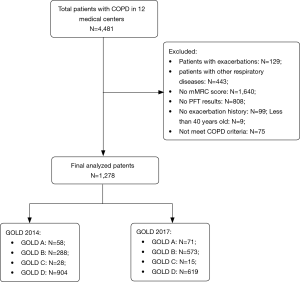
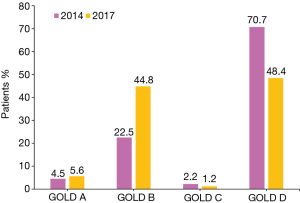
According to the GOLD 2014 and 2017 classification criteria, the distribution of the 1,278 COPD patients across groups A–D was 58 (4.5%), 288 (22.5%), 28 (2.2%), 904 (70.7%) and 71 (5.6%), 573 (44.8%), 15 (1.2%), 619 (48.4%), respectively (Figure 2). The number of patients was lowest in group C and highest in group D according to both the GOLD 2014 and 2017 classification criteria. The new classification led to 13 patients (46.4% of group C) being reclassified from group C to group A, and 285 patients (31.5% of group D) being reclassified from group D to group B. Overall, 298 patients (32.0% of groups C–D) in high-risk groups were reclassified to low-risk groups.
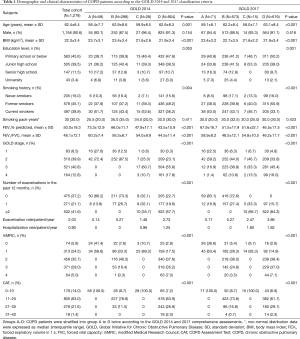
The mean (± SD) age of the patients was 62.4±8.4 years, and most were male (90.6%). The mean BMI of the patients was 22.3±3.4 kg/m2. Further, 85.1% had not been educated to higher than junior high school, and most were either former (45.1%) or current (38.9%) smokers (Table 1). Based on the airflow limitation results, the patients were most commonly classified as having moderate (39.9%) or severe (40.8%) COPD. Over 30% of patients had not experienced an exacerbation in the last year, while 41.6% had experienced two or more exacerbations. The mean annual rate of exacerbations and hospitalizations in the previous year were 2.03 and 0.90, respectively. The most common mMRC grade was 2 (35.7%) and approximately two-thirds of patients had CAT scores of 11–20.
Full table
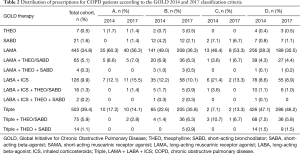
The most frequently prescribed treatment regimens were triple inhaled treatment (39.4%) involving an ICS, a long-acting beta-agonist (LABA), and a long-acting muscarinic antagonist (LAMA), followed by LAMA-only treatment (34.8%) (Table 2). A total of 736 patients (57.6%) were treated with ICS.
Full table
Demographic and clinical characteristics according to GOLD 2014 and 2017 classification criteria
According to both the GOLD 2014 and 2017 classification criteria, the patients in groups B and D (with more symptoms) were older than those in groups A and C (with fewer symptoms) (Table 1). The patients in groups C–D were more likely to have lower BMI. About 50% of the patients in group D were former smokers, whereas the majority of patients in groups A, B, and C were current smokers. There were no significant differences among the four groups with respect to sex or pack-years of smoking.
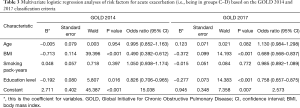
Multivariate logistic regression analyses were performed with the following factors as independent variables: age, BMI, education level, and pack-years of smoking. The dependent variable was high or low risk of exacerbation (groups C–D vs. A–B). According to both the GOLD 2014 and 2017 classification criteria, independent risk factors for high risk of exacerbation (groups C–D) were lower BMI (P<0.001) and education level (P<0.05) (Table 3).
Full table
Pharmacotherapy according to GOLD 2014 and 2017 classification criteria
Among the patients from groups A and C according to both the GOLD 2014 and 2017 classification criteria, the most frequently prescribed therapy was LAMA-only treatment (Table 2). Triple treatment (ICS + LAMA + LABA) was the most frequently prescribed regimen among the patients in the old and new group D (47.1% and 46.2%, respectively). ICS therapy, in dual (ICS + LABA) or triple treatment regimens, was prescribed across all GOLD groups. In particular, ICS therapy was used in 38.2% and 54.3% of the old and new group B patients, respectively, and 29.3% and 33.8% of the old and new group A patients, respectively. Use of short-acting bronchodilator monotherapy was more common in groups B and C stratified by both the GOLD 2014 and 2017 classification criteria. The proportion of patients prescribed theophylline monotherapy or a combination of other drugs was very small.
Comparisons of reclassified and non-reclassified patients
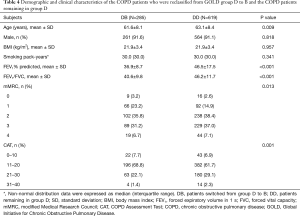
More than one-third of the patients formerly classified in group D with FEV1% predicted <50% had no history of frequent acute exacerbation. Now, this group of patients were reassigned to group B. Compared with the patients who were reclassified from group D to B (subgroup DB), the patients remaining in group D (subgroup DD) were older (P=0.009) and had poorer pulmonary function (P<0.001) and higher mMRC grades and CAT scores (P=0.013 and 0.001, respectively) (Table 4). There were no statistically significant differences regarding other variables (sex, BMI, and pack-years of smoking).
Full table
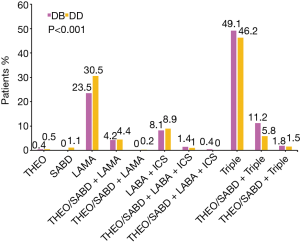
The patients in subgroup DB were prescribed fewer LAMA-only regimens (23.5% vs. 30.5%) but more triple treatment regimens (49.1% vs. 46.2%) than the patients in subgroup DD (Figure 3). A total of 205 (71.9%) of the 285 patients in subgroup DB were treated with ICS.
Given the small number of patients in groups A and C, no further analysis was undertaken.
Discussion
According to the GOLD 2014 and 2017 classification criteria, this study investigated the categorization, demographics, clinical characteristics, and pharmacotherapy of COPD patients attending outpatient clinics in Hunan, China. More than one-third of the patients in high-risk groups were reclassified to low-risk groups. Low BMI and education level were identified as independent risk factors for high risk of exacerbation (groups C–D). The most frequently prescribed regimens were triple treatment and LAMA-only treatment. ICS therapy was prescribed across all groups A–D, which, according to GOLD 2017, implies overtreatment in a considerable number of patients in groups A–B. However, a notable change was that the decreased number of patients in high-risk groups might affect therapeutic decisions to a lesser degree. We believe that our findings present the first comparison of the treatment regimens of Chinese COPD patients classified according to both the GOLD 2014 and 2017 classification criteria. In addition, compared with a national cross-sectional survey conducted on outpatients nearly 10 years ago in China (20), our patient sample is more recent and hence more representative of the present situation.
We showed that the GOLD 2017 classification criteria reclassified many COPD patients to low-risk groups. This reclassification is consistent with previous reports (15-20). Recently, a study of 834 patients with COPD at 67 Hungarian secondary care outpatient clinics reported that 66% and 72% of the patients in groups C and D (GOLD 2016) were reclassified to groups A and B (GOLD 2017), respectively (23). In addition, in our study, according to the 2014 GOLD classification criteria, more individuals were categorized in group D, which is similar to the results of previous studies of COPD patients recruited from hospital clinics (14,24). In contrast, in a COPD cohort identified from the general population, group A became predominant (25). When classified by the GOLD 2017 classification criteria, we found groups B and D to be the most prevalent groups. Similarly, in the POPE cohort (recruited in a secondary care setting), there was a high proportion of patients in groups B and D (18). In contrast, a study that enrolled outpatients with a wide range of COPD severity showed that group A accounted for the largest proportion of patients (15). The distribution of patients in groups A–D may be substantially different depending on the different populations and study methods.
Another interesting finding was that groups A and C were relatively small. Several previous studies have also reported that group C is the smallest group (15,16,19), suggesting that it is unusual for COPD patients who are at high risk of exacerbations to not report many symptoms. However, among the previous studies, groups A and C were both small in the POPE cohort only (18). The subjects in our study were recruited from tertiary hospitals and most patients had marked symptoms, which led to a limited number of patients in groups A and C. Moreover, it is known that the current cut-off points for the CAT scores and mMRC grades do not lead to exactly the same classification (14,26). Our analyses confirmed that the questionnaire employed to determine categorization based on symptoms had a major impact on group sizes. In our study, patients with a CAT score ≥10 or mMRC grade ≥2 accounted for 86.0% and 69.7%, respectively. Thus, many patients were categorized into groups B and D because of having higher CAT scores. Of note, the patients in our study whose education levels were generally low might have refused to seek medical help until developing severe symptoms, which also decreased the proportions in groups A and C.
In our analyses, low BMI was a risk factor for high risk of exacerbation. Studies have shown that low BMI is an important risk factor for the development of COPD (27,28). Furthermore, a prospective study at a tertiary hospital reported that with increasing COPD stage, the BMI decreased progressively (29). A study of data on 2,265 subjects from a large pulmonary function laboratory database showed that with increasing BMI, subjects had significant improvements in the FEV1/FVC ratio, and this effect was greatest in patients with the most severe airway obstruction (30). In the COPDGene study, researchers also reported that a higher BMI was associated with lower lung volumes and higher expiratory airflows (31). As a result, early intervention for COPD patients with low BMI may improve outcomes.
In our study, low education level was a risk factor for high risk of exacerbation. A previous large-scale population-based cross-sectional survey reported that lower education level is a risk factor of COPD (32). The 5th Korea National Health and Nutrition Examination Survey reported that the development of COPD occurred at a 5.36-time higher rate in subjects whose education level was primary school or less compared to subjects who whose education level was college or higher (33). Moreover, the inhalation technique of COPD patients without face-to-face training is mostly unsatisfactory, especially regarding patients who are poorly educated (34).
Treatment varied significantly among the GOLD groups, and we observed that adherence to the GOLD 2014 or 2017 treatment recommendations was far from optimal. GOLD 2017 recommends using triple treatment only in group D patients who develop further exacerbations on LAMA + LABA (1), and GOLD 2014 recommends triple treatment as the first-line therapy only in group D patients (10). However, the ICS + LAMA + LABA regimen was used in a substantial number of our patients regardless of their GOLD group. Although ICS therapy is recommended only for patients in groups C–D (1,10), a considerable proportion of low-risk patients were prescribed ICS + LABA or ICS in combination with other drugs. Similarly, overtreatment has been observed in an increasing number of clinical trials (18,19,35). However, a physician may prescribe medication for a patient based on their overall clinical judgment rather than guidelines. It is important to note that when evaluating adherence by clinicians to GOLD guidelines, it is necessary to compare different GOLD versions in patients that received and did not receive the recommended treatment.
In 2013, Wedzicha et al. reported that a LAMA + LABA fixed-dose combination was much better at preventing moderate-to-severe COPD exacerbations than LAMA-only treatment (36). Recent data has suggested that LAMA + LABA is also superior to ICS + LABA at preventing exacerbations (37). In our study, no patients were treated with LABA-only or LABA + LAMA regimens. A primary reason was that indacaterol, the single LABA, was not available in most hospitals in Hunan, and many physicians were more likely to empirically use LAMA-only or ICS + LABA regimens (compared with LABA-only or LAMA + LABA regimens), which were more widely available and affordable to all patients who needed them. However, ICS should be prescribed only in specific situations, mainly because of the risk of pneumonia, a risk not seen with LABA + LAMA (37,38).
To the best of our knowledge, our study is the first to compare the demographic and clinical characteristics of COPD patients who were reclassified from GOLD group D to B and patients remaining in group D. The patients who were reclassified from group D to B were younger and had fewer symptoms than the patients who remained in group D. The GOLD 2017 treatment recommendations recommended initial therapy with LABA and/or LAMA for patients in group B. Although the patients who were reclassified from group D to B had poor pulmonary function without frequent exacerbations, we found that their prescriptions were usually triple inhaled therapies rather than bronchodilators. More than 70% of these patients were treated with ICS. However, for these reclassified patients, dual bronchodilation therapy should be the first-line therapy and ICS should be prescribed less frequently. Harlander et al. proposed that for patients without co-existing asthma who are reclassified from group D to B, ICS should be discontinued if the blood eosinophil count is <300 cells/µL (39). Therefore, the reclassification should lead to a decrease in the use of ICS. As a result, ICS-related complications such as pneumonia or pulmonary tuberculosis could be reduced.
Our study has several limitations. First, because the patients in our study were all recruited from outpatient clinics at tertiary hospitals and treated by physicians, our results may not be generalizable to untreated patients or those with milder symptoms. Second, groups A and C had only a small number of patients, which may have limited the power of the analyses regarding these groups and affected the accuracy and reliability of our analysis. Third, the small number of women in this cohort—possibly because women in China seldom smoke and are far more reluctant to seek medical advice than men—makes our results more difficult to extrapolate to women. However, the study reflects the reality of COPD outpatients in Hunan, China. Fourth, the cost and availability of the different drugs in the hospitals surveyed should also be considered, for example, LABA was not available in most hospitals in Hunan. Fifth, the GOLD 2017 guidelines were released in November 2016, which may have affected the physicians’ treatment decisions regarding patients recruited in 2017 and 2018, leading to underestimation of the effects of the GOLD 2017 guidelines. Despite these limitations, our study objectively assessed the situation in Hunan and involved recently recruited patients, so the results reflect the current characteristics of COPD patients and prescribing practices.
Conclusions
This comparative analysis of the GOLD 2014 and 2017 classification criteria confirms that the latter reclassifies large numbers of patients in groups C–D to groups A–B. The risk of exacerbation increased with decreased BMI or education level. The patients that were reclassified from group D to B were younger and had fewer symptoms compared with those that remained in group D. In addition, there was a misalignment between GOLD treatment recommendations and actual treatments for COPD patients. Physicians should reexamine treatment patterns for patients reclassified into low-risk groups. Further studies are needed to assess the impact of the GOLD 2017 classification criteria and their ability to predict outcomes such as future exacerbations and mortality.
Acknowledgements
The authors would like to acknowledge the substantial contributions to conception and design, acquisition of data from Shan Cai, MD of the Second Xiangya Hospital of Central South University and Yuqin Zeng, DO of the Second Xiangya Hospital of Central South University. The authors also would like to acknowledge the substantial contributions to acquisition of data from investigators in the Hunan Occupational Disease Prevention and Treatment Hospital, the Affiliated Hospital of Guilin Medical University, the Second People’s Hospital of Guilin, the Third Hospital of Changsha, the First Traditional Chinese Medicine Hospital of Changde, the First People’s Hospital of Huaihua, the Central Hospital of Xiangtan, the Traditional Chinese Medicine Hospital of Zhuzhou, the Traditional Chinese Medicine Hospital of Yueyang, the First People’s Hospital of Yueyang and the Central Hospital of Hengyang. The authors also acknowledge all the patients who paryicipated in this study.
Funding: This study was supported by the National Science Foundation of China (No. 81400032, No. 81600031, and No. 81873410) and the National Key Clinical Specialty Construction Projects of China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (No. ChiCTR-POC-17010431), and written informed consent was obtained from all participants.
References
- GOLD Executive Committee, Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2017 REPORT). Available online: . Accessed Nov 2016.https://goldcopd.org/
- Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health 2015;5:020415. [Crossref] [PubMed]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117-71. [Crossref] [PubMed]
- Csikesz NG, Gartman EJ. New developments in the assessment of COPD: early diagnosis is key. Int J Chron Obstruct Pulmon Dis 2014;9:277-86. [PubMed]
- Maio S, Baldacci S, Carrozzi L, et al. Respiratory symptoms/diseases prevalence is still increasing: a 25-yr population study. Respir Med 2016;110:58-65. [Crossref] [PubMed]
- Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 2005;366:1875-81. [Crossref] [PubMed]
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741-50. [Crossref] [PubMed]
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706-17. [Crossref] [PubMed]
- GOLD Executive Committee, Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (Revised 2011). Available online: . Accessed Dec 2011.https://goldcopd.org/
- GOLD Executive Committee, Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (Updated 2014). Available online: . Accessed Jan 2014.https://goldcopd.org/
- Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [Crossref] [PubMed]
- Jones P, Miravitlles M, van der Molen T, et al. Beyond FEV1 in COPD: a review of patient-reported outcomes and their measurement. Int J Chron Obstruct Pulmon Dis 2012;7:697-709. [Crossref] [PubMed]
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-38. [Crossref] [PubMed]
- Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med 2013;1:43-50. [Crossref] [PubMed]
- Cabrera López C, Casanova Macario C, Marin Trigo JM, et al. Comparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on Grouping and Outcomes. Am J Respir Crit Care Med 2018;197:463-9. [Crossref] [PubMed]
- Faner R, Noell G, Badia JR, et al. Distribution, temporal stability and association with all-cause mortality of the 2017 GOLD groups in the ECLIPSE cohort. Respir Med 2018;141:14-9. [Crossref] [PubMed]
- Tan WC, Bourbeau J, Aaron SD, et al. Global Initiative for Chronic Obstructive Lung Disease 2017 Classification and Lung Function Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2018;197:670-3. [Crossref] [PubMed]
- Tudoric N, Koblizek V, Miravitlles M, et al. GOLD 2017 on the way to a phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) Cohort. Eur Respir J 2017;49. [Crossref] [PubMed]
- Marçôa R, Rodrigues DM, Dias M, et al. Classification of Chronic Obstructive Pulmonary Disease (COPD) according to the new Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017: Comparison with GOLD 2011. COPD 2018;15:21-6. [Crossref] [PubMed]
- Sun L, Chen Y, Wu R, et al. Changes in definition lead to changes in the clinical characteristics across COPD categories according to GOLD 2017: a national cross-sectional survey in China. Int J Chron Obstruct Pulmon Dis 2017;12:3095-102. [Crossref] [PubMed]
- Goossens LM, Leimer I, Metzdorf N, et al. Does the 2013 GOLD classification improve the ability to predict lung function decline, exacerbations and mortality: a post-hoc analysis of the 4-year UPLIFT trial. BMC Pulm Med 2014;14:163. [Crossref] [PubMed]
- Yang H, Xiang P, Zhang E, et al. Predictors of exacerbation frequency in chronic obstructive pulmonary disease. Eur J Med Res 2014;19:18. [Crossref] [PubMed]
- Bikov A, Horváth A, Tomisa G, et al. Changes in the Burden of Comorbidities in Patients with COPD and Asthma-COPD Overlap According to the GOLD 2017 Recommendations. Lung 2018;196:591-9. [Crossref] [PubMed]
- Agustí A, Rennard S, Edwards LD, et al. Clinical and prognostic heterogeneity of C and D GOLD groups. Eur Respir J 2015;46:250-4. [Crossref] [PubMed]
- Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med 2012;186:975-81. [Crossref] [PubMed]
- Jones PW, Adamek L, Nadeau G, et al. Comparisons of health status scores with MRC grades in COPD: implications for the GOLD 2011 classification. Eur Respir J 2013;42:647-54. [Crossref] [PubMed]
- Ding Y, Xu J, Yao J, et al. The analyses of risk factors for COPD in the Li ethnic group in Hainan, People's Republic of China. Int J Chron Obstruct Pulmon Dis 2015;10:2593-600. [Crossref] [PubMed]
- Zhou Y, Wang D, Liu S, et al. The association between BMI and COPD: the results of two population-based studies in Guangzhou, China. COPD 2013;10:567-72. [Crossref] [PubMed]
- Gupta SS, Gothi D, Narula G, et al. Correlation of BMI and oxygen saturation in stable COPD in Northern India. Lung India 2014;31:29-34. [Crossref] [PubMed]
- O'Donnell DE, Deesomchok A, Lam YM, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest 2011;140:461-8. [Crossref] [PubMed]
- Abston E, Comellas A, Reed RM, et al. Higher BMI is associated with higher expiratory airflow normalised for lung volume (FEF25-75/FVC) in COPD. BMJ Open Respir Res 2017;4:e000231. [Crossref] [PubMed]
- Caballero A, Torres-Duque CA, Jaramillo C, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest 2008;133:343-9. [Crossref] [PubMed]
- Oh H, Lee YE. Prevalence and risk factors of chronic obstructive pulmonary disease among nonsmokers: Fifth Korea National Health and Nutrition Examination Survey (2010-2012). Osong Public Health Res Perspect 2016;7:385-93. [Crossref] [PubMed]
- Pothirat C, Chaiwong W, Phetsuk N, et al. Evaluating inhaler use technique in COPD patients. Int J Chron Obstruct Pulmon Dis 2015;10:1291-8. [Crossref] [PubMed]
- Gunen H, Yilmaz M, Aktas O, et al. Categorization of COPD patients in Turkey via GOLD 2013 strategy document: ALPHABET study. Int J Chron Obstruct Pulmon Dis 2015;10:2485-94. [Crossref] [PubMed]
- Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med 2013;1:199-209. [Crossref] [PubMed]
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:2222-34. [Crossref] [PubMed]
- Iannella H, Luna C, Waterer G. Inhaled corticosteroids and the increased risk of pneumonia: what's new? A 2015 updated review. Ther Adv Respir Dis 2016;10:235-55. [Crossref] [PubMed]
- Harlander M, Barrecheguren M, Turel M, et al. Should patients switched from D to B in the GOLD 2017 classification be discontinued from inhaled corticosteroids? COPD 2017;14:465-8. [Crossref] [PubMed]


