
The impact of low forced vital capacity on behavior restrictions in a population with airflow obstruction
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease characterized by persistent respiratory symptoms and airflow limitation (1). Exertional dyspnea is the most common symptom of COPD and often restricts the performance of physical activities of daily life, leading to disability and reduced health-related quality of life (2-4). Indeed, physical activity levels are substantially lower in stable COPD patients than in healthy subjects (5,6), and reduced physical activity puts COPD patients at greater risk of hospitalization (7-12) and mortality (8,11-13). The degree of physical activity restrictions is associated with severity of COPD (5,14) but reduced physical activity is also observed in COPD patients with early-stages of the disease (15) and leads to further decline of lung function (16).
A recent study evaluating COPD patients revealed that the population with a restrictive pattern, defined as a normal forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio and low FVC, had respiratory symptoms and reduced exercise performance, established by a six-minute walking test, at a level similar to moderate COPD patients (17). Accumulating findings indicate that low FVC is significantly associated with various comorbid conditions, including aging, cardiovascular diseases, metabolic syndrome, and obesity (18-20), which may further affect physical inactivity. However, to our knowledge, no studies have yet evaluated the impact of coexisting low FVC on physical activity in a population with airflow obstruction (AO), particularly within a similar degree of AO. Thus, this study aimed to evaluate the distribution of low FVC and the association between low FVC and behavior restrictions in subjects with AO who participated in the Korea National Health and Nutritional Examination Survey (NHANES), particularly based on the severity of AO.
Methods
Study population
The Korea NHANES is a cross-sectional, national representative survey of the non-institutionalized South Korean population conducted periodically by the Korean Ministry of Health and Welfare using a stratified, multistage clustered probability sampling design. Stratification is conducted based on the 13 areas of Korea (seven metropolitan cities and six provinces; administrative unit (e.g., dong, eup, and, myeon which are Korean units); and housing type e.g., apartment or other types of housings). Sampling units were defined on the basis of household registries, including geographic area, sex, and age group (21). All members of each selected household were asked to participate in the survey, and the participation rate between 2007 and 2015 ranged from 71.2% to 82.8%. The Korea NHANES includes a health interview, a nutritional survey, and a health exam including a pulmonary exam, all conducted by trained investigators in a specially equipped mobile examination center. For this study, we used data from the Korea NHANES IV [2007–2009], the Korea NHANES V [2010–2012], and the Korea NHANES VI [2013–2015] pertaining to participants aged 40 to 79 years who had undergone a pulmonary function test and a blood test. Spirometry was performed according to the recommendations of the American Thoracic Society/European Respiratory Society (22). Absolute values of FEV1 and FVC were obtained, and the percentage predicted of values (% predicted) for FEV1 and FVC were calculated from equations obtained in a representative Korean sample (23). The study sample consisted of 2,437 men and 908 women (total 3,345 participants). The Korea NHANES was reviewed and approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention, and all participants provided written informed consent. Detailed methods of the Korea NHANES, including survey representativeness and response rate, are available elsewhere (21).
Spirometry-defined AO was defined as pre-bronchodilator FEV1/FVC <70% (24). Low FVC was defined as FVC <80% predicted. Severity of AO was classified as mild (FEV1 ≥80% predicted), moderate (50%≤ FEV1 <80% predicted), or severe-to-very severe (FEV1 <50% predicted) (1).
Existence of behavior restrictions was ascertained through the Korea NHANES question, “Are you limited in anyway in any activity because of any impairment or health problem?”. Diabetes mellitus (DM) was defined as use of glucose-lowering medications, a blood glucose level ≥126 mg/dL, a hemoglobin A1c level ≥6.5%, or a self-reported physician diagnosis. Hypertension was defined as the use of antihypertensive medication or a systolic blood pressure ≥140 mmHg, or a diastolic blood pressure ≥90 mmHg, or a self-reported physician diagnosis. Dyslipidemia was defined as use of lipid-lowering medications, a high-density lipoprotein-cholesterol level <40 mg/dL in men or <50 mg/dL in women, a triglyceride level ≥150 mg/dL, or a self-reported physician diagnosis. Stroke, myocardial infarction or angina, asthma, and osteoporosis were defined as self-reported physician diagnoses (25).
Statistical analysis
Statistical analysis used the survey commands of Stata (release 14.1; StataCorp LP, College Station, TX, USA) to account for survey weights and the complex sampling design. We calculated the prevalence rate and standard error (SE) to compare the characteristics of subjects with AO and low FVC to those with normal FVC. We used Poisson regression to estimate adjusted prevalence ratio (aPR) and 95% confidence interval (CI) after adjusting for age, sex, smoking status, body mass index (BMI; kg/m2), and number of comorbidities. Two-sided P values of 0.05 were considered to be statistically significant.
Results
Study population
The baseline characteristics of the subjects with AO in this study are summarized in Table 1. Of the study population, 1,545 (46.4%), 1,639 (48.7%), and 161 (4.9%) subjects had mild, moderate, and severe airflow limitations, respectively. In comparison to the subjects with mild or moderate AO, those with severe-to-very severe AO were more likely to be current smokers (P=0.017), to have lower BMI values (P=0.023), and to have bronchial asthma (P<0.001). The prevalence of osteoporosis was lowest in subjects with mild AO and highest in subjects with severe AO (P=0.014).
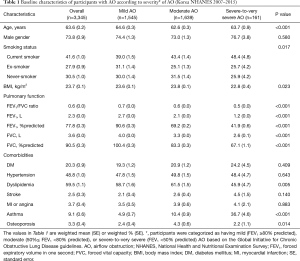
Full table
Prevalence and clinical characteristics of subjects with low FVC according to severity of AO
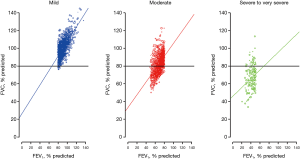
As shown in Figure 1, the weighted prevalence of subjects with low FVC was 0.9% (n=12), 35.5% (n=591), and 85.1% (n=131) in the mild, moderate, and severe-to-very severe AO groups, respectively.
The clinical characteristics of subjects with low FVC versus those with normal FVC, organized by the severity of AO, are summarized in Table 2. Among subjects with mild AO, those with low FVC were more likely to be older (P<0.001), to have a larger waist circumference (P<0.001), and to have a higher BMI (P=0.005) compared to those with normal FVC. Except for DM (P=0.029) and stroke (P=0.007), which were more prevalent in subjects with low FVC, there were no significant differences in prevalence of comorbidities between subjects with low FVC and those with normal FVC. Regarding laboratory findings, serum creatinine level was higher in subjects with low FVC compared to those with normal FVC (P=0.001), while there were no significant differences in white blood cell counts and hemoglobin levels between the two groups.
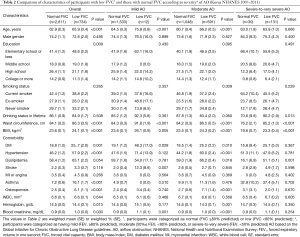
Full table
Among subjects with moderate AO, those with low FVC were more likely to be older (P<0.001) and, to be never-/ex-smokers (P=0.009), to have a larger waist circumference (P<0.001), and to have a higher BMI (P<0.001) compared to those with normal FVC. Regarding comorbidities, DM (P=0.010), hypertension (P<0.001), dyslipidemia (P=0.018), and osteoporosis (P<0.001) were more frequent among subjects with low FVC versus those with normal FVC. In addition, while hemoglobin level was lower in subjects with low FVC compared to those with normal FVC (P=0.001), serum creatinine was higher in subjects with low FVC compared to those with normal FVC (P<0.001).
In subjects with severe-to-very severe AO, whereas there were no significant differences in terms of age and smoking history, those with low FVC were more likely to have a larger waist circumference (P<0.001) and a higher BMI (P<0.001) than those with normal FVC. With respect to comorbidities, the presence of dyslipidemia (P=0.001) was more frequent among subjects with low FVC compared to those with normal FVC.
Association between presence of low FVC and behavior restrictions according to severity of AO
As shown in Table 3, the presence of low FVC was associated with behavior restrictions among all subjects with AO, in both crude (PR, 1.87; 95% CI, 1.55–2.25) and adjusted models (aPR, 1.72; 95% CI, 1.43–2.06). When the subjects were stratified according to AO severity, there was no significant association between presence of low FVC and behavior restrictions among subjects with mild or severe-to-very-severe AO. In contrast, low FVC in the moderate AO group was independently associated with behavior restrictions (PR, 1.86; 95% CI, 1.45–2.39), which persisted even after adjusting for age, sex, smoking status, BMI, and number of comorbidities (aPR, 1.65; 95% CI, 1.27–2.13). Further adjustment for FEV1% predicted did not alter this association (aPR, 1.41; 95% CI, 1.06–1.87).

Full table
Discussion
In this study, prevalence of low FVC in subjects with AO increased with severity of AO, showing a significant correlation between FEV1 and FVC. While less than 1% of patients in the mild AO group had low FVC, more than 80% of those in the severe-to-very severe AO group had low FVC, indicating a limitation of low FVC as a biomarker in these populations. However, in the moderate AO group, about one-third of individuals had low FVC while two-thirds had normal FVC. The clinical characteristics were also substantially different according to presence or absence of low FVC in the moderate AO group; subjects with low FVC were more likely to be older and never-/ex-smokers, to have a larger waist circumference and a higher BMI, and to demonstrate comorbidities such as DM, hypertension, dyslipidemia, and osteoporosis compared to those with normal FVC. Most importantly, low FVC was an independent factor associated with behavior restriction in subjects with moderate AO, whereas there was no significant association between low FVC and behavior restrictions in either the mild or severe AO group.
There are several explanations for the association of low FVC with behavior restrictions in subjects with AO. First, low FVC might represent a distinctive metabolic phenotype characterized by a large waist circumference and the presence of DM and dyslipidemia, which was inversely associated with physical activity (26). Previous studies have shown that the prevalence of metabolic syndrome is significantly increased as FVC is decreased, with abdominal obesity playing a critical role (20,27). Another potential explanation is the association between low FVC and lung hyperinflation. Previous research suggests that physical activity and symptoms in COPD subjects are more closely associated with lung hyperinflation than with FEV1 (28-30). In these studies, not only dynamic hyperinflation, but also static hyperinflation were consistently shown to be related to poor quality of life, reduced exercise capacity, and increased mortality (29-31). In some subjects with obstructive lung disease, low FVC can be exhibited despite increased lung volume with hyperinflation due to premature peripheral airway closure during expiration (32). For example, a study exploring the relationship between lung hyperinflation and COPD outcomes showed that a considerable proportion of subjects with hyperinflation (defined as residual volume/total lung capacity >40%) had reduced FVC, while most subjects without hyperinflation had normal FVC. These findings jointly suggest that low FVC associated with hyperinflation might result in reduced physical activity in subjects with AO (28-31,33). However, since the current study did not measure lung volumes, further investigations are needed to confirm our hypothesis. Taken together, the presence of low FVC reflecting these complicated morbidities might be an indicator of reduced physical activity even among subjects with AO.
When the association between low FVC and behavior restriction was analyzed in subgroups according to AO severity, there was no significant association between low FVC and behavior restrictions in mild and severe AO groups. The effect of low FVC might have been compensated for by the relatively preserved lung function in subjects with mild AO. Likewise, the additional impact of low FVC might not be critical in subjects with severe-to-very-severe AO, since these patients are already severely affected by their very low FEV1. In addition, given that the positive association was observed in the entire study population and the number of subjects with low FVC (n=12) in the mild AO group and those with normal FVC (n=30) in the severe AO group were small, this phenomenon might simply be due to the lack of statistical power.
Physical inactivity is an independent predictor of poor outcomes in COPD patients; it is associated with exacerbation-related hospitalization (7-12) and mortality (11-13). In addition, persistent physical inactivity over time is related to rapid progression of exercise intolerance and muscle depletion (4). However, since physical inactivity might be correctable with physical rehabilitation, patient education, and psychosocial support (34), it is important to identify COPD subjects with decreased physical activity. Despite its importance, physical inactivity may be underappreciated by clinicians, probably due to the lack of a simple method to elucidate patients’ activity levels (34). Although physical activity can be assessed by observing the patient directly, evaluating energy expenditure during movement, administering physical activity questionnaires, and reviewing a patient’s activity diary, these methods are usually time-consuming and impractical in daily routine practice (35), necessitating a useful biomarker for predicting behavior restrictions in the population with AO. In this view, our study provided informative data that low FVC is independently associated with behavior restrictions in subjects with AO, especially those with moderate AO.
Our study has several limitations that need to be considered when interpreting our results. First, the cross-sectional design of this study limits the assessment of the causal relationship between low FVC and behavior restrictions. Therefore, the causal inference must be evaluated in subsequent studies. Second, we used a nationwide representative sample of Koreans, which might not be generalizable to subjects in different countries or of other ethnicities. Third, behavior restriction was determined by asking a single question to survey participants. Subjects with underlying AO might not report any behavior restriction if they have already adopted to an inactive life style. Thus, this dichotomized question might have underestimated behavior restriction decreased our ability to detect the degree of behavior restriction. Further studies with more objective measurement of behavior restriction are needed to investigate the impact of low FVC on behavior restrictions. Finally, although we exclusively included subjects with AO, mixed restrictive ventilatory impairment might have contributed to low FVC. Differentiating various conditions, such as interstitial lung disease, disease of pleura, or neuromuscular disease, usually requires measurement of diffusing capacity, lung volume by plethysmography, and chest imaging. Since these tests were not performed in the Korea NHANES, we could not assess the presence of restrictive ventilatory impairment in our study.
In conclusion, presence of low FVC may represent a clinically distinctive phenotype among the population with AO, characterized by old age, lighter smoking history, obesity, and metabolic comorbidities. In addition, low FVC was independently associated with behavior restrictions even after adjusting for confounding factors, which suggests its potential role as a biomarker reflecting physical inactivity in subjects with AO, especially in those with moderate AO.
Acknowledgements
None.
Footnote
Conflicts of Interest: HY Park has received lecture fees from AstraZeneca, Novartis, and Boehringer-Ingelheim. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention and written informed consent was obtained from all patients.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD 2017. 2017 [accessed 2017 Mar 16]. Available online: http://goldcopd.org/
- Arne M, Lundin F, Boman G, et al. Factors associated with good self-rated health and quality of life in subjects with self-reported COPD. Int J Chron Obstruct Pulmon Dis 2011;6:511-9. [Crossref] [PubMed]
- Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J 2010;36:292-300. [Crossref] [PubMed]
- Waschki B, Kirsten AM, Holz O, et al. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:295-306. [Crossref] [PubMed]
- Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171:972-7. [Crossref] [PubMed]
- Vorrink SN, Kort HS, Troosters T, et al. Level of daily physical activity in individuals with COPD compared with healthy controls. Respir Res 2011;12:33. [Crossref] [PubMed]
- Pitta F, Troosters T, Probst VS, et al. Physical activity and hospitalization for exacerbation of COPD. Chest 2006;129:536-44. [Crossref] [PubMed]
- Garcia-Aymerich J, Lange P, Serra I, et al. Time-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: an application of the marginal structural model. Ann Epidemiol 2008;18:775-83. [Crossref] [PubMed]
- Benzo RP, Chang CC, Farrell MH, et al. Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration 2010;80:10-8. [Crossref] [PubMed]
- Garcia-Aymerich J, Farrero E, Felez MA, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003;58:100-5. [Crossref] [PubMed]
- Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772-8. [Crossref] [PubMed]
- Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012;142:338-46. [Crossref] [PubMed]
- Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011;140:331-42. [Crossref] [PubMed]
- Jehn M, Schmidt-Trucksass A, Meyer A, et al. Association of daily physical activity volume and intensity with COPD severity. Respir Med 2011;105:1846-52. [Crossref] [PubMed]
- Van Remoortel H, Hornikx M, Demeyer H, et al. Daily physical activity in subjects with newly diagnosed COPD. Thorax 2013;68:962-3. [Crossref] [PubMed]
- Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med 2007;175:458-63. [Crossref] [PubMed]
- Vaz Fragoso CA, McAvay G, Van Ness PH, et al. Phenotype of spirometric impairment in an aging population. Am J Respir Crit Care Med 2016;193:727-35. [Crossref] [PubMed]
- Godfrey MS, Jankowich MD. The vital capacity is vital: Epidemiology and clinical significance of the restrictive spirometry pattern. Chest 2016;149:238-51. [Crossref] [PubMed]
- Guerra S, Sherrill DL, Venker C, et al. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax 2010;65:499-504. [Crossref] [PubMed]
- Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med 2009;179:509-16. [Crossref] [PubMed]
- Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol 2014;43:69-77. [Crossref] [PubMed]
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. [Crossref] [PubMed]
- Choi J, K., Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis 2005;58:230-42. [Crossref]
- Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc 2015;12:1788-95. [Crossref] [PubMed]
- Lee H, Shin SH, Gu S, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med 2018;16:178. [Crossref] [PubMed]
- Kim J, Tanabe K, Yokoyama N, et al. Association between physical activity and metabolic syndrome in middle-aged Japanese: a cross-sectional study. BMC Public Health 2011;11:624. [Crossref] [PubMed]
- Kang HK, Park HY, Jeong BH, et al. Relationship between forced vital capacity and framingham cardiovascular risk score beyond the presence of metabolic syndrome: The fourth Korea National Health and Nutrition Examination Survey. Medicine (Baltimore) 2015;94:e2089. [Crossref] [PubMed]
- Krieger BP. Hyperinflation and intrinsic positive end-expiratory pressure: less room to breathe. Respiration 2009;77:344-50. [Crossref] [PubMed]
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc 2006;3:176-9. [Crossref] [PubMed]
- Lahaije AJ, van Helvoort HA, Dekhuijzen PN, et al. Resting and ADL-induced dynamic hyperinflation explain physical inactivity in COPD better than FEV1. Respir Med 2013;107:834-40. [Crossref] [PubMed]
- Shin TR, Oh YM, Park JH, et al. The prognostic value of residual volume/total lung capacity in patients with chronic obstructive pulmonary disease. J Korean Med Sci 2015;30:1459-65. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948. [Crossref] [PubMed]
- Garcia-Rio F, Lores V, Mediano O, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med 2009;180:506-12. [Crossref] [PubMed]
- Spruit MA, Pitta F, McAuley E, et al. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:924-33. [Crossref] [PubMed]
- Troosters T, van der Molen T, Polkey M, et al. Improving physical activity in COPD: towards a new paradigm. Respir Res 2013;14:115. [Crossref] [PubMed]


