Evidence based imaging strategies for solitary pulmonary nodule
Introduction
Lung cancer is the leading cause of cancer related death throughout the world (1). Lung cancer screening programs are being investigated in the United States, Japan, and other countries with low-dose helical/multi-detector CT. Despite the controversy on its cost-effectiveness (2), evidences suggest that early detection of lung cancer allow more timely therapeutic intervention and thus a more favorable prognosis for the patient (3-5). Solitary pulmonary nodule (SPN) is defined as a rounded opacity 3 cm in diameter surrounded by lung parenchyma (6). Lesions larger than 3 cm are called masses and are often malignant (6). On CT, nodules can be solid, semisolid (mixed attenuation), or ground-glass attenuation. Traditionally, chest radiography provides basic information about SPN. Nowadays, patients with SPN detected on radiographs are likely to undergo early CT scan (7). The majority of smokers who undergo thin-section CT have been found to have small lung nodules, most of which are smaller than 7 mm in diameter (8,9). However, the clinical importance of these small nodules differs substantially from that of larger nodules detected on chest radiographs, in that the vast majority are benign. This has been highlighted in several recent publications on CT screening for lung cancer (10-15). In the past, multiple follow-up examinations over a 2-year period, including CT follow-up at 3, 6, 12, 18, and 24 months, were recommended when such nodules are detected incidentally (16,17). The policy increases radiation burden for the affected population (18-21). This editorial will present the current evidence based imaging strategies for SPN.
Morphologic assessment of solitary pulmonary nodule (SPN)
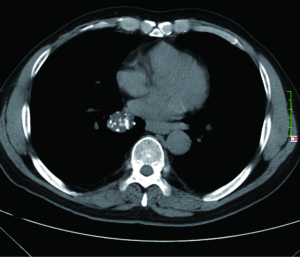
The most common intrapulmonary malignant lesions are metastases and primary bronchopulmonary carcinoma. All histological types of cancer may give rise to pulmonary nodules, but adenocarcinoma is the most frequent (22). Eighty percent of benign nodules are granulomas or intrapulmonary lymph nodes, 10% are hamartomas and 10% are other rarer benign lesions (23,24). Nodule features such as shape, edge characteristics, cavitation, and location have not yet been found to be accurate for distinguishing benign from malignant nodules (Figure 1) (25,26). Features favoring benignity include evidence of stability for two years or more, small nodule size, smooth demarcated margins, and certain pattern of calcifications (central dense, diffuse, laminated or popcorn). Clustering of multiple nodules in a single location in the lung tend to favor an infectious process, although a dominant nodule with adjacent small satellite nodules can be seen in primary lung cancer (27,28). A laminated or central pattern is typical of a granuloma, whereas a classic “popcorn” pattern is most often seen in hamartomas (Figure 2) (24). In approximately half the cases of hamartoma, high-resolution CT can show a definitive pattern of fat and cartilage (29). Fat content suggests a hamartoma or occasionally a lipoid granuloma or lipoma (30). Calcification patterns that are stippled or eccentric have been associated with cancer. Another benign entity is rounded atelectasis. Diagnosis for rounded atelectasis can be made as it has specific diagnostic morphological features including subpleural location, curved course of blood vessels into the opacity, and evidence of pleural disease (Figure 3) (31). When nodules considered as benign no further investigation is necessary.
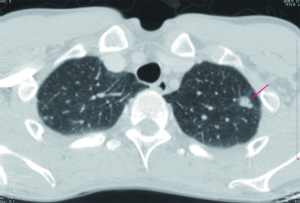
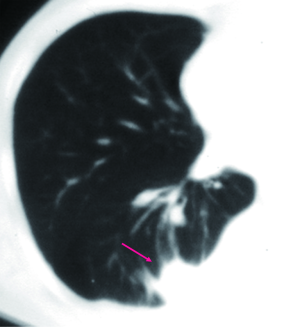
SPNs with irregular, spiculated margins, or lobulated contours, are typically associated with malignancy. Two patterns of the margins of a nodule are relatively specific for cancer. One is the corona radiata sign, consisting of very fine linear strands extending 4 to 5 mm outward from the nodule; they have a spiculated appearance on plain radiographs (Figures 4,5). A scalloped border is associated with an intermediate probability of cancer. Although most SPNs with smooth, well-defined margins are benign, these features are not diagnostic of benignity. A total of 21% of malignant nodules had well-defined margins (29).
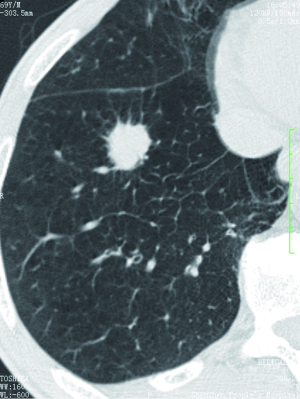
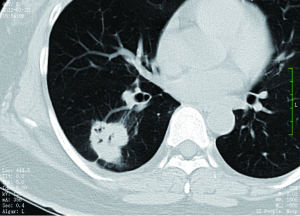
For a single nodule, upper lobe location increases the likelihood of malignancy, because primary lung cancers are more common in the upper lobes (32). On the other hand, small, irregular, benign subpleural opacities, presumably due to scarring, are extremely common in the apical areas in older patients. Triangular or ovoid circumscribed nodules 3-9 mm in diameter adjacent to pleural fissures commonly represent intrapulmonary lymph nodes (33).
In general, purely linear or sheetlike lung opacities are unlikely to represent neoplasms and do not require follow-up (34).
Approaches to indeterminate solitary pulmonary nodule (SPN)
When SPN is considered to be indeterminate in the initial exam, the risk factor of the patients should be evaluated. Increasing patient age generally correlates with increasing likelihood of malignancy. On the other hand, lung cancer is uncommon in patients younger than 40 years and is rare in those younger than 35 years (<1% of all cases) (35). The relative risk for developing lung carcinoma in male smokers was about 10 times that in nonsmokers (36). For heavy smokers, the risk was 15-35 times greater (37). Also, the cancer risk for smokers increases in proportion to the degree and duration of exposure to cigarette smoke (38). Other established risk factors include exposure to asbestos, uranium, and radon (39-41). A history of lung cancer in first-degree relatives is also a risk factor, and strong evidence for a specific lung cancer susceptibility gene has been discovered recently (42,43). A history of cancer can greatly increase the likelihood of a nodule being malignant (44). Low-risk individuals are <50 years old and have <20 pack-year smoking history. Moderate-risk group is defined as age >50 and >20 pack-year smoking history or second hand exposure, and no additional risk factor (random exposure, occupational exposure, cancer history, family history, or lung cancer).
The positive relationship of lesion size to likelihood of malignancy has been clearly demonstrated (10-15). The standard size value used is an average of the largest and smallest cross-sectional diameters of the most representative area of the nodule. In a meta-analysis of eight large screening trials, the prevalence of malignancy depended on the size of the nodules, ranging from 0% to 1% for nodules 5 mm or smaller, 6% to 28% for those between 5 and 10 mm, and 64% to 82% for nodules 20 mm or larger. Even in smokers, the percentage of all nodules smaller than 4 mm that will eventually turn into lethal cancers is very low (<1%), whereas for those in the 8-mm range the percentage is approximately 10-20%. The 2005 Fleischner Society guideline (Table 1) stated that at least 99% of all nodules 4 mm or smaller are benign and because such small opacities are common on thin-section CT scans, follow-up CT in every such case is not recommended; in selected cases with suspicious morphology or in high-risk subjects, a single follow-up scan in 12 months should be considered (45). This protocol could result in a few indolent cancers being missed, the number of such instances would be extremely small relative to the reduction in the number of unnecessary studies (45).

Full table
The increased use of CT screening for lung cancer led to an increase in identification of early lesions such as adenocarcinoma in situ (AIS, histopathologically ≤30 mm, noninvasive lepidic growth, which at CT is usually nonsolid; Figure 6) and minimally invasive adenocarcinoma (MIA, histopathologically ≤30 mm and predominantly lepidic growth that has 5-mm or smaller invasion, which at CT is mainly nonsolid but may have a central solid component of up to approximately 5 mm; Figure 7) (22). These lesions should not be regarded as conventional invasive adenocarcinomas and can be observed rather than surgically resected (22).
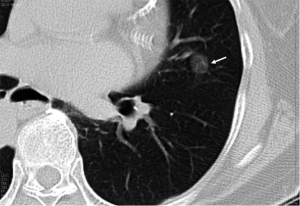
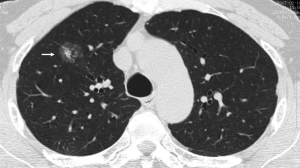
When the nodule is 5-9 mm in diameter, approximately 6% of cases showed interval nodule growth detectable on 4-8 month follow-up scans (11). For these nodules the best strategy is surveillance. The timing of these control examinations is given in Table 1. This varies according to the nodule size (4-6, or 6-8 mm) and type of patients, specifically at low or high risk of malignancy concerned. Noncalcified nodules larger than 8 mm diameter can bear a substantial risk of malignancy (Figure 8) (46). In the case of nodules larger than 8 mm, additional options such as contrast material-enhanced CT, positron emission tomography (PET), percutaneous needle biopsy, and thoracoscopic resection or videoassisted thoracoscopic resection can be considered (13). A common aspect of invasive adenocarcinoma of the lung is metastasis to the brain (47,48). Its probability is a function of the size of the primary tumor, at least for tumors in the 20-60 mm size range. For node-negative invasive adenocarcinoma of the lung, a 20 mm primary lesion has been found to show a 14% probability of brain metastatic disease, progressing nearly linearly to a 60 mm primary node-negative lesion showing a 64% probability of brain metastatic disease (47).
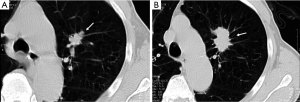
In certain clinical settings, such as a patient presenting with neutropenic fever, the presence of a nodule may indicate active infection, and short-term imaging follow-up may be appropriate. Previous CT scans, chest radiographs, and other pertinent imaging studies should be obtained for comparison whenever possible, as they may serve to demonstrate either stability or interval growth of the nodule in question.
In a patient with known primary malignancy, lung nodules, regardless of being solitary or multiple, would be deemed suspicious for metastases. Pertinent factors will include the site, cell type, and stage of the primary tumor and whether early detection of lung metastases will affect care.
CT follow-up for solitary pulmonary nodule (SPN)
The volume doubling time (DT) for malignant bronchogenic tumors is rarely less than a month or more than a year. A nodule that was not present on a radiograph obtained less than two months before the current image is therefore not likely to be malignant. The “doubling time” (DT) of a nodule can be calculated using the following formula:
Where Vi is the initial volume of the nodule, Vf the final volume, t the time interval between observations and ln the logarithmic value. This formula is based on an exponential model of nodule growth (23). Note that a 5-mm nodule with a DT of 60 days will reach a diameter of 20.3 mm in 12 months, whereas a similar nodule with a DT of 240 days would reach a diameter of only 7.1 mm in the same period.
It has been considered that stability of nodule size for over 2 years for solid pulmonary nodules suggest benign nature. Recently, the radiologic-pathologic correlation of pure ground-glass attenuation nodules and mixed attenuation nodules with the histologic spectrum of pulmonary adenocarcinoma was described (49). Small purely ground-glass opacity (nonsolid) nodules that have malignant histopathologic features tend to grow very slowly, with a mean volume DT on the order of two years (50). Solid cancers, on the other hand, tend to grow more rapidly, with a mean volume DT on the order of 6 months. The growth rate of partly solid nodules tends to fall between these extremes, and this particular morphologic pattern is predictive of adenocarcinoma (46,51,52). Bronchoalveolar cell carcinomas and typical carcinoids occasionally appear to be stable for two or more years (53). Longer follow-up intervals are appropriate for nonsolid (ground-glass opacity) and very small opacities (50,51). Even if malignant, a nonsolid nodule that is smaller than 6 mm will probably not grow perceptibly in much less than 12 months (50,51). Currently, though the dictum that two-year stability on plain-film radiography indicates a benign process should be used with caution (54), it is still reasonable to use two-year stability on high-resolution CT as a practical guideline for predicting a benign process.
Hasegawa et al. (50) reported an analysis of the growth rates of small lung cancers detected during a 3-year mass screening program. They classified nodules as ground-glass opacity, as ground-glass opacity with a solid component, or as solid. Mean volume DTs were 813, 457, and 149 days, respectively. In addition, the mean volume DT for cancerous nodules in nonsmokers was significantly longer than that for cancerous nodules in smokers. Authors of a number of other series have confirmed similar findings and have estimated the median tumor DTs, assuming a constant growth rate to be in the 160-180-day range (55,56). Authors of all of these reports, however, recognize wide variations, and in one study 22% of tumors had a volume DT of 465 days or more (56).
The use of stability as an indicator of a benign process is predicated on accurate measurement of growth and thus on the resolution of the imaging technique used. The accurate measurement of growth in subcentimeter nodules can be problematic. A doubling in volume of a sphere corresponds to an increase of only 26% of its diameter according to the formula V=4/3πr3 where r is the radius. Therefore, it may be difficult to evaluate an increase or decrease in the axial diameter of a nodule between two successive CT examinations, or even of no value for small nodules less than or equal to 5 mm in size. In fact, a nodule of 5 mm which doubles in volume will only increase in diameter by 1.25 mm. Revel et al. determined that two-dimensional measurements obtained with electronic calipers were unreliable as a basis for distinguishing benign from malignant solid nodules in the 5-15-mm size range (57). Some authors compared diameter and cross-sectional area measurement with volume measurement in the assessment of lung tumour growth with serial CT. They demonstrated that growth assessment of lung tumours measuring less than 3 cm on CT serial CT scans with non-volumetric measurements frequently disagrees with growth assessment with volumetric measurements, and the three-dimensional measurements are more reliable (58-62). Volume measurement requires specific image analysis software, which allows segmentation and three-dimensional reconstruction of the nodule in order to appreciate the variations in morphology, and to automatically calculate the volume. Goodman et al. have raised additional caution in applying volumetric measurements, because the overall variability between scans in vivo is still substantial with wide confidence limits of 13.1%±26.6% (63). For this reason, it is recommended to act on variations in nodule volume of 20%. A variation of <20% should not be considered as significant as it could be due to the method of measurement. Factors that affect the reproducibility of nodule volume measurement on CT include nodule size at detection, examination technique, nodule relationship to adjacent structures, underlying lung disease, and patient factors such as phase of respiration and cardiac motion (58).
For optimal CT evaluation of subsolid pulmonary nodules, thin sections are advisable. Changes in nodule attenuation can also be assessed. For malignant subsolid nodules, measuring an increase in attenuation at serial CT examinations appears to be less subject to variability than measures of diameter or volume (64). Also, when subjective visual assessment of a nonsolid nodule at serial thin-section CT examinations suggests either possible but not definite enlargement of the nodule or possible but not definite development of a solid component, then increasing “mass” (mean volume multiplied by attenuation) may help identify early invasive growth, the increasing solid component corresponding to progression of local malignant invasion (64). For example, an increase of 100 HU in attenuation of a nonsolid nodule has been described as representing an approximately 10% increase in tumor volume (65). During surveillance of ground glass nodules, the appearance of a soft-tissue component is a highly suspicious sign of malignancy, even if the overall size of the nodule remains stable or diminishes (66).
An important aspect of adenocarcinomas of the lung is that, for small, solitary, early-stage tumors, the size of the invasive component-as measured histologically-is an independent predictor of survival (67). For part-solid lesions evaluated by using CT for tumor size, the size of the solid portion may be more predictive of prognosis than total size that includes the nonsolid dimension (67-69). A recent multicenter study in Japan has indicated that, for resected stage IA (T1N0M0) adenocarcinoma of the lung, disease-free survival correlated with solid tumor size but not with “whole tumor size” that included a nonsolid (ground-glass) component (70). For part-solid stage IA adenocarcinoma of the lung, an extensive nonsolid component is a favorable prognostic sign (22).
SPN can also appear as focal nodular areas of increased lung attenuation, including both well and poorly defined lesions, through which normal parenchymal structures, including airways and vessels, can be visualized. This appearance typically is referred to a ground-glass nodule. The term “subsolid” nodules are used to emphasize that both pure ground-glass nodules and part-solid ground-glass nodules. It is necessary to establish lesions as true ground-glass nodule by using contiguous thin CT sections (1 mm thickness) whenever possible to avoid the pitfall of interpreting lesions as subsolid on thick sections (typically 5 mm) when they are actually solid. The 2013 Fleischner Society recommendation for the management of Subsolid Pulmonary Nodules is shown in Table 2 (71).
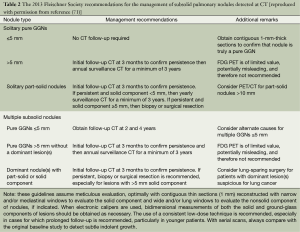
Full table
A low-dose, thin-section, unenhanced technique should be used, with limited longitudinal coverage, when follow-up of a lung nodule is the only indication for the CT examination. Malignant probability for nodules can be calculated using the software available on the website of Dr Gurney (http://www.chestx-ray.com).
Contrast-enhanced CT for solitary pulmonary nodule (SPN)
After administration of iodinated contrast material intravenously with power injection (300 mg/mL at 2 mL/sec), nodular enhancement of less than 15 HU is strongly predictive of benignity (Figure 9); whereas enhancement of more than 20 HU, reflecting presence of tumour neo-vascularisation, is indicative of malignancy (Figure 10). Results from a large multicenter study found that contrast-enhanced CT has a sensitivity of 98% and a specificity of 58% when using a cutoff of 15 Hounsfield units for enhancement (72). A recent meta-analysis of ten dynamic CT studies reported pooled sensitivity of 93%, specificity of 76%, positive predictive value (PPV) of 80% and negative predictive value (NPV) of 95% for SPN characterization (73). Higher accuracy is reported for dynamic enhancement evaluation on helical CT, by analyzing combined wash-in and washout characteristics. Malignant nodules show greater washout of contrast enhancement (74). Dual-energy CT imaging has also been used in several studies to evaluate nodules with similar diagnostic accuracy (75,76). Limitations of contrast-enhanced CT relate to its false positive results for malignancy caused by inflammatory lesions; and measurement error that can occur in evaluation of small nodules. Given that measurement of the density is difficult for heterogeneous lesions and those less than 1 cm in diameter, in practice contrast-enhanced CT only yields reliable information for homogenous nodules equal to or above 8 mm in diameter.
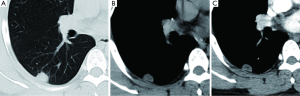

Positron emission tomography (PET) and combined PET-CT
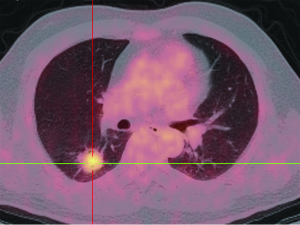
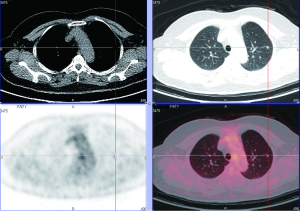
PET, using 18-fluorine fluoro-deoxyglucose (18F-FDG), a D-glucose analogue labeled with radio-isotope, can quantify the rate of glucose metabolism by cells, thereby detecting presence of metabolically active tissue. Malignant nodules consist of metabolically active cells that have higher uptake of glucose due to over-expression of glucose transporter protein (Figure 11). FDG is trapped and accumulates within these cells, as the radio-labeled glucose analogue is phosphorylated once but not metabolized further (77). A meta-analysis reported pooled sensitivity of 96.8% and specificity of 77.8% for malignant nodules for 8F-FDG PET technique alone (7). Integration of CT and PET results in an improved accuracy, with 97% sensitivity and 85% specificity, for differentiating malignant from benign SPNs (78). Kim et al. found that visual interpretation by experienced radiologist or nuclear medicine specialist is sufficient, if not superior, for characterizing SPN, and quantitative analysis (using 2.0 as cut-off SUVmax) did not improve accuracy (78). There is growing evidence that using threshold SUVmax to differentiate malignant from benign lesions is unrealistic, and SUVmax of 2.5 should not be embraced as a magic threshold.
Higher FDG uptake in lung cancer as measured by standardized uptake value (SUV) analysis is associated with aggressive cancers and shorter survival (79,80). On the other hand, lower levels of FDG uptake often correspond to histologically and clinically less aggressive tumour behavior (Figure 12) (81). An added value of PET-CT is the detection of other unexpected metabolically active lesion and/or lymphadenopathy to support probable diagnosis of SPN being a primary or secondary malignancy (82). Up to 14% of patients otherwise eligible for surgery have occult extrathoracic disease on whole body PET imaging (83). On the other hand, the sensitivity and specificity of CT scans for detecting mediastinal lymph-node involvement are 55% to 88% and 76% to 85%, respectively (84). The sensitivity and specificity of PET in the presence of abnormal lymph nodes on CT scanning are 94% and 82%, respectively. In one prospective study, the diagnostic accuracy of CT was 64%, that of PET 88%, and that of the combination of CT and PET 96% (85).
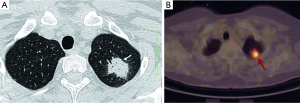
In most studies, the sensitivity of 18F-FDG PET-CT tends to be higher than its specificity for assessment of SPN. Many benign conditions, such as granulomatous (for example histoplasmosis or tuberculosis) and inflammatory processes, can mimic malignant nodules and produce false-positive results (Figure 13) (86). On the other hand, false negative results for SPN characterization on PET-CT can occur in three main settings: small lesion size, low tumor metabolic activity, and hyperglycemia. Small lesions (<1 cm) are challenging due to limited spatial resolution of PET, which is approximately 7 mm for modern scanners (7). Some highly differentiated malignant tumors have relatively little metabolic activity and low rate of proliferation, resulting in false-negative PET-CT. FDG PET is falsely negative in around 50% of patients with bronchioloalveolar carcinoma (87), or adenocarcinoma in situ (88-90). In addition, metastasis from certain primary malignancy, such as renal cell carcinoma, testicular or prostate cancer, may show little FDG tracer accumulation and may even be undetectable on PET-CT (91). False negative FDG PET-CT scans may also occur in patients with hyperglycemia (77). Some authors have proposed dual time point FDG-PET imaging, using the change in SUVmax between early and delayed scans to help differentiate benign and malignant SPNs (92). However, the role of dual time point PET imaging (DTPI) has been disputed by some authors (93). A meta-analysis on diagnostic performance of dual time point FDG-PET imaging in assessing lung nodules reported similar sensitivity and specificity to single time point FDG-PET (94). Further studies are needed to clarify this point.
The major obstacles to widespread use of PET-CT are limited availability and high costs. In the United States, with increasing availability of PET scanners and the reimbursement for SPN evaluation and lung cancer staging with PET scans being supported by Medicare, PET-CT has become much more common. In the United Kingdom, the National Institute for Clinical Excellence (NICE) currently recommends 18F-FDG PET for investigation of SPNs in cases where a biopsy is not possible or has failed, depending on nodule size, position and CT characterization (95).
Magnetic resonance imaging (MRI)
Use of MRI in the evaluation of pulmonary nodules has so far been limited. Faster imaging sequences and techniques to mitigate artefacts have allowed for detection of smaller nodules (6 to 10 mm) with a sensitivity of almost 95% (96). For nodules >1 cm, contrast-enhanced dynamic MRI has been shown to be comparable to CT for distinguishing benign from malignant nodules (97-99). A meta-analysis of six dynamic contrast-enhanced MR studies reported pooled sensitivity of 94%, which is comparable to dynamic contrast enhanced CT, but with higher pooled specificity of 79% (73). Mori et al. found diffusion weighted imaging (DWI) was more specific for SPNs than FDG PET, due to fewer false positives for active inflammation, which does not affect diffusion of water molecules (100). An obvious advantage of MRI is this technique does not involve radiation. Further development in sequencing, scanners and coils, adaptation of parallel and sparse MR imaging will speed up the scan time (101,102), making MRI a realistic tool for the imaging of lung, particularly for lung cancer screen.
Computer-aided detection and diagnosis
Modern CT generates a large number of images that must be read by radiologists/physicians. This may lead to ‘‘information overload’’ for the radiologists/physicians. They may miss some cancers during their interpretation of CT images (52,103). Therefore, a computer-aided diagnosis (CAD) scheme for detection of lung nodules in CT images has been investigated as a tool for lung cancer detection. CAD is often categorized into two major groups: computer-aided detection (CADe) and computer-aided diagnosis (CADx). CADe focuses on a detection task, i.e., detection (or localization) of lesions in medical images. CADx focuses on a diagnosis (characterization) task, e.g., distinction between benign and malignant lesions, and classification among different lesion types (103,104). The use of CADe systems improves the performance of radiologists in the detection process of pulmonary nodules (105-107). It has been reported that CADe systems improved the sensitivity of radiologists in detecting small nodules on CT scans higher than that with conventional double reading (108). It has been also shown that observer’s nodule detection remained imperfect, and that a maximum-intensity-projection processing technique reduced the number of overlooked small nodules, particularly in the central lung (109).
Various approaches have been proposed for CADe schemes for lung nodules in CT. Sensitivities for detection of lung nodules in CT range from 70% to 95%, with from a few to 70 false positives (FP) per case. Figure 14 illustrates CADe outputs on a CT image of the lungs (110,111). Major sources of FPs are various-sized lung vessels. Major sources of false negatives are ground glass nodules, nodules attached to vessels, and nodules attached to the lung wall (i.e., juxtapleural nodules). Ground glass nodules are difficult to detect, because they are subtle, of low-contrast, and have ill-defined boundaries.
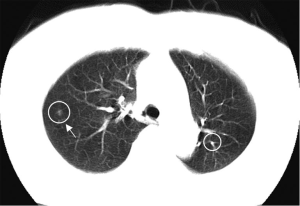
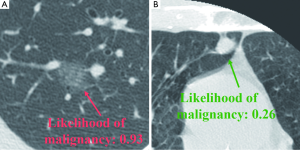
A number of researchers developed CADx schemes distinguishing malignant nodules from benign nodules on CT images automatically and/or determining the likelihood of malignancy for the detected nodules. Figure 15 illustrates CADx outputs on malignant and benign lung nodules on high-resolution CT images (112,113). The performance of CADx schemes ranges from area under the receiver-operating-characteristic curve (AUC) values of 0.85 to 0.95 (107). Overall, the sensitivities of CADe schemes are relatively high, but the number of FPs is high compared to radiologists’ performance (107). Further improvement in specificity is necessary in future research.
Transthoracic fine-needle aspiration biopsy
Transthoracic fine-needle aspiration biopsy may be optimised by the presence of an onsite cytopathologist at the time of biopsy, allowing repeated sampling if insufficient cells are obtained. For those teams not have an onsite cytopathologist, coaxial cutting needles are recommended which yield more voluminous biopsy samples. This technique improves the accuracy of diagnosis without significant increase in the complication rate. CT may be useful in biopsy planning by specifying lesion depth and the point of the needle in order to aid the approach, and to avoid the needle path traversing a bulla or fissure. Although the minimum size varies according to the expertise of the radiologist, a diameter of at least 7 mm is usually required. For malignant lesions, transthoracic fine-needle aspiration biopsy offer the sensitivity of 80% to 95% and the specificity is 50% to 88%. For lesions that are less than 2 cm in diameter, transthoracic fine-needle aspiration biopsy has a sensitivity of more than 60 percent for detecting a malignant process (114). However, the false negative rate is 3% to 29% (115).
The Achilles heel of needle biopsy is negative biopsy result for malignancy without specific benign lesion diagnosis does not exclude malignancy. In addition, the technique has limited ability in producing specific diagnosis of benign lesions. SPN with high clinical suspicion of malignancy that is operable, the best approach is surgical resection as needle biopsy does not influence management (positive result confirms the high clinical suspicion and will be followed by resection, negative result does not exclude malignancy and the lesion has to be removed surgically because of the high clinical suspicion) so the result whether positive or negative does not alter the patient’s management. For inoperable SPN, needle biopsy is justified to confirm the histology. Complication rates of needle biopsy are higher than those for bronchoscopy. Pneumothorax and haemorrhage are seen in 5-30% of cases, although in most cases, treatment is not required.
Bronchoscopy
If the nodule is linked to a narrowed or obstructed bronchus, a bronchus is visible within the nodule or an endobronchial lesion is detected on CT, then “bronchoscopy targeting” to the appropriate level is recommended. In such a case, the CT examination can optimize the approach to biopsy, and guide direct transbronchial biopsy. The sensitivity of bronchoscopy for detecting a malignant process in a SPN ranges from 20% to 80%, depending on the size of the nodule, its proximity to the bronchial tree, and the prevalence of cancer in the study population (116). For nodules that are less than 1.5 cm in diameter, the sensitivity is 10%, and for those that are 2.0 to 3.0 cm in diameter, it is 40% to 60% (116,117). When CT reveals a bronchus leading to the lesion, bronchoscopy may have a 70% sensitivity (118).
Conclusions
This paper highlights the importance of avoiding excessive patient irradiation caused by unnecessary follow-up CTs (45). The radiation dosage for a chest varies between 1-10 mSv, while that of whole body FDG-PET/CT is 10-30 mSv. More details on medical X-ray radiation risk can be found at http://www.xrayrisk.com/. After the publication of the Fleischner Society guideline in 2005, it was reported more frequent CT follow-ups than necessary are still being performed (119,120). The policy of low-dose, thin-section, with limited longitudinal coverage for follow-up CTs is not always carried out (119,120).
Information from the morphologic appearance of the nodule can be combined with clinical risk information to produce an overall probability for malignancy. If this probability can be set sufficiently low, strategies that include observing nodules for interval change can be advocated. The patient’s age and the presence of comorbid conditions influence management recommendations. The patient’s preference is also a very important factor, especially if the potential difference among strategies is small. It is also highly practical that management approaches differ from institution to institution because of the varying prevalence of lung disease in different parts of the world, varying skill levels of operators, and varying availability of equipments.
Acknowledgements
The authors thank Dr. Junqing Xu, Xijing Hospital, The Forth Military Medical University, Xi’an, China, for helpful discussion.
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Goulart BH, Bensink ME, Mummy DG, et al. Lung cancer screening with low-dose computed tomography: costs, national expenditures, and cost-effectiveness. J Natl Compr Canc Netw 2012;10:267-75. [PubMed]
- Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet 1998;351:1242-5. [PubMed]
- Heelan RT, Flehinger BJ, Melamed MR, et al. Non-small-cell lung cancer: results of the New York screening program. Radiology 1984;151:289-93. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [PubMed]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [PubMed]
- Swensen SJ. CT screening for lung cancer. AJR Am J Roentgenol 2002;179:833-6. [PubMed]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Henschke CI, Yankelevitz DF, Naidich DP, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [PubMed]
- Swensen SJ, Jett JR, Hartman T, et al. Screening for lung cancer with CT: Mayo Clinic experience. Radiology 2003;226:756-61. [PubMed]
- Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [PubMed]
- Henschke CI, Naidich DP, Yankelevitz DF, et al. Early Lung Cancer Action Project: initial findings on repeat screening. Cancer 2001;92:153-9. [PubMed]
- Diederich S, Wormanns D, Lenzen H, et al. Screening for asymptomatic early bronchogenic carcinoma with low dose CT of the chest. Cancer 2000;89:2483-4. [PubMed]
- Ost D, Fein AM, Feinsilver SH. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [PubMed]
- Tan BB, Flaherty KR, Kazerooni EA, et al. The solitary pulmonary nodule. Chest 2003;123:89S-96S. [PubMed]
- Mayo JR, Alrich J, Muller NL. Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology 2003;228:15-21. [PubMed]
- Imhof H, Schibany N, Ba-Ssalamah A, et al. Spiral CT and radiation dose. Eur J Radiol 2003;47:29-37. [PubMed]
- Kalra MK, Maher MM, Toth TL, et al. Strategies for CT radiation dose optimization. Radiology 2004;230:619-28. [PubMed]
- Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004;231:440-5. [PubMed]
- Austin JH, Garg K, Aberle D, et al. Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 2013;266:62-71. [PubMed]
- Beigelman-Aubry C, Hill C, Grenier PA. Management of an incidentally discovered pulmonary nodule. Eur Radiol 2007;17:449-66. [PubMed]
- Kradin RL, Spirn PW, Mark EJ. Intrapulmonary lymph nodes: clinical, radiologic, and pathologic features. Chest 1985;87:662-7. [PubMed]
- Brandman S, Ko JP. Pulmonary nodule detection, characterization, and management with multidetector computed tomography. J Thorac Imaging 2011;26:90-105. [PubMed]
- Zhao F, Yan SX, Wang GF, et al. CT features of focal organizing pneumonia: an analysis of consecutive histopathologically confirmed 45 cases. Eur J Radiol 2014;83:73-8. [PubMed]
- Heitzman ER, Markarian B, Raasch BN, et al. Pathways of tumor spread through the lung: radiologic correlations with anatomy and pathology. Radiology 1982;144:3-14. [PubMed]
- Yano M, Arai T, Inagaki K, et al. Intrapulmonary satellite nodule of lung cancer as a T factor. Chest 1998;114:1305-8. [PubMed]
- Erasmus JJ, Connolly JE, McAdams HP, et al. Solitary pulmonary nodules. I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000;20:43-58. [PubMed]
- Gaerte SC, Meyer CA, Winer-Muram HT, et al. Fat-containing lesions of the chest. Radiographics 2002;22:S61-78. [PubMed]
- Batra P, Brown K, Hayashi K, et al. Rounded atelectasis. J Thorac Imaging 1996;11:187-97. [PubMed]
- Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: an epidemiologic inquiry. J Natl Cancer Inst 1984;72:1271-5. [PubMed]
- Hyodo T, Kanazawa S, Dendo S, et al. Intrapulmonary lymph nodes: thin-section CT findings, pathological findings, and CT differential diagnosis from pulmonary metastatic nodules. Acta Med Okayama 2004;58:235-40. [PubMed]
- Takashima S, Sone S, Li F, et al. Small solitary pulmonary nodules (≤1 cm) detected at population-based CT screening for lung cancer: reliable high-resolution CT features of benign lesions. AJR Am J Roentgenol 2003;180:955-64. [PubMed]
- Gadgeel SM, Ramalingam S, Cummings G, et al. Lung cancer in patients < 50 years of age: the experience of an academic multidisciplinary program. Chest 1999;115:1232-6. [PubMed]
- U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, Ga: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1982.
- Guyatt GH, Newhouse MD. Are active and passive smoking harmful? determination of causation. Chest 1985;88:445-51. [PubMed]
- Bach PB, Kattan M, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst 2003;95:470-8. [PubMed]
- Lee PN. Relation between exposure to asbestos and smoking jointly and the risk of lung cancer. Occup Environ Med 2001;58:145-53. [PubMed]
- Gottlieb LS, Husen LA. Lung cancer among Navajo uranium miners. Chest 1982;81:449-52. [PubMed]
- Field RW, Steck DJ, Smith BJ, et al. Residential radon gas exposure and lung cancer: the Iowa Radon Lung Cancer Study. Am J Epidemiol 2000;151:1091-102. [PubMed]
- Mayne ST, Buenconsejo J, Janerich D. Familial cancer history and lung cancer risk in United States nonsmoking men and women. Cancer Epidemiol Biomarkers Prev 1999;8:1065-9. [PubMed]
- Bailey-Wilson JE, Amos CI, Pinney SM, et al. A major lung cancer susceptibility locus maps to chromosome 6q2325. Am J Hum Genet 2004;75:460-74. [PubMed]
- Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology 2000;217:257-61. [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [PubMed]
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [PubMed]
- Mujoomdar A, Austin JH, Malhotra R, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882-8. [PubMed]
- Komaki R, Cox JD, Stark R. Frequency of brain metastasis in adenocarcinoma and large cell carcinoma of the lung: correlation with survival. Int J Radiat Oncol Biol Phys 1983;9:1467-70. [PubMed]
- Godoy MC, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. Radiology 2009;253:606-22. [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [PubMed]
- Aoki T, Nakata H, Watanabe H, et al. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol 2000;174:763-8. [PubMed]
- Li F, Sone S, Abe H, et al. Lung cancers missed at low-dose helical CT screening in a general population: comparison of clinical, histopathologic, and imaging findings. Radiology 2002;225:673-83. [PubMed]
- Fein AM, Feinsilver SH, Ares CA. The solitary pulmonary nodule: a systemic approach. In: Fishman AP. eds. Pulmonary diseases and disorders. 3rd ed. New York: McGraw-Hill, 1998.
- Yankelevitz DF, Henschke CI. Does 2-year stability imply that pulmonary nodules are benign? AJR Am J Roentgenol 1997;168:325-8. [PubMed]
- Usuda K, Sato Y, Sagawa M, et al. Tumor doubling time and prognostic assessment of patients with primary lung cancer. Cancer 1994;74:2239-44. [PubMed]
- Winer-Muram HT, Jennings SG, Tarver RD, et al. Volumetric growth rate of stage I lung cancer prior to treatment: serial CT scanning. Radiology 2002;223:798-805. [PubMed]
- Revel MP, Bissery A, Bienvenu M, et al. Are two-dimensional CT measurements of small noncalcified pulmonary nodules reliable? Radiology 2004;231:453-8. [PubMed]
- Kostis WJ, Yankelevitz DF, Reeves AP, et al. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology 2004;231:446-52. [PubMed]
- Revel MP, Lefort C, Bissery A, et al. Pulmonary nodules: preliminary experience with three-dimensional evaluation. Radiology 2004;231:459-66. [PubMed]
- Yankelevitz DF, Gupta R, Zhao B, et al. Small pulmonary nodules: evaluation with repeated CT - preliminary experience. Radiology 1999;212:561-6. [PubMed]
- Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217:251-6. [PubMed]
- Wormanns D, Kohl G, Klotz E, et al. Volumetric measurements of pulmonary nodules at multi-row detector CT: in vivo reproducibility. Eur Radiol 2004;14:86-92. [PubMed]
- Goodman LR, Gulsun M, Washington L, et al. Inherent variability of CT lung nodule measurements in vivo using semiautomated volumetric measurements. AJR Am J Roentgenol 2006;186:989-94. [PubMed]
- de Hoop B, Gietema H, van de Vorst S, et al. Pulmonary ground-glass nodules: increase in mass as an early indicator of growth. Radiology 2010;255:199-206. [PubMed]
- Zhang L, Yankelevitz DF, Carter D, et al. Internal growth of nonsolid lung nodules: radiologic-pathologic correlation. Radiology 2012;263:279-86. [PubMed]
- Kakinuma R, Ohmatsu H, Kaneko M, et al. Progression of focal pure ground-glass opacity detected by low-dose helical computed tomography screening for lung cancer. J Comput Assist Tomogr 2004;28:17-23. [PubMed]
- Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol 2009;33:462-9. [PubMed]
- Sakao Y, Miyamoto H, Sakuraba M, et al. Prognostic significance of a histologic subtype in small adenocarcinoma of the lung: the impact of nonbronchioloalveolar carcinoma components. Ann Thorac Surg 2007;83:209-14. [PubMed]
- Sakao Y, Nakazono T, Tomimitsu S, et al. Lung adenocarcinoma can be subtyped according to tumor dimension by computed tomography mediastinal-window setting: additional size criteria for clinical T1 adenocarcinoma. Eur J Cardiothorac Surg 2004;26:1211-5. [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Prognostic significance of using solid versus whole tumor size on high-resolution computed tomography for predicting pathologic malignant grade of tumors in clinical stage IA lung adenocarcinoma: a multicenter study. J Thorac Cardiovasc Surg 2012;143:607-12. [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [PubMed]
- Swensen SJ, Viggiano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000;214:73-80. [PubMed]
- Cronin P, Dwamena BA, Kelly AM, et al. Solitary pulmonary nodules: meta-analytic comparison of cross sectional imaging modalities for diagnosis of malignancy. Radiology 2008;246:772-82. [PubMed]
- Jeong YJ, Lee KS, Jeong SY, et al. Solitary pulmonary nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology 2005;237:675-83. [PubMed]
- Chae EJ, Song JW, Seo JB, et al. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology 2008;249:671-81. [PubMed]
- Kang MJ, Park CM, Lee CH, et al. Dual-energy CT: clinical applications in various pulmonary diseases. Radiographics 2010;30:685-98. [PubMed]
- Sim YT, Poon FW. Imaging of solitary pulmonary nodule-a clinical review. Quant Imaging Med Surg 2013;3:316-26. [PubMed]
- Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007;48:214-20. [PubMed]
- Bryant AS, Cerfolio RJ. The maximum standardized uptake values on integrated FDG-PET/CT is useful in differentiating benign from malignant pulmonary nodules. Ann Thorac Surg 2006;82:1016-20. [PubMed]
- Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18) F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging 2010;37:1087-94. [PubMed]
- Kwee TC, Basu S, Saboury B, et al. A new dimension of FDG-PET interpretation: assessment of tumor biology. Eur J Nucl Med Mol Imaging 2011;38:1158-70. [PubMed]
- Sahiner I, Vural GU. Positron emission tomography/computerized tomography in lung cancer. Quant Imaging Med Surg 2014;4:195-206. [PubMed]
- Schmid RA, Hillinger S, Bruchhaus H, et al. The value of positron emission tomography (FDG PET) in detecting extrathoracic metastases in non-small cell lung cancer. Am J Respir Crit Care Med 1998;157:A256.
- Martini N, Flehinger BJ, Zaman MB, et al. Prospective study of 445 lung carcinomas with mediastinal lymph node metastases. J Thorac Cardiovasc Surg 1980;80:390-9. [PubMed]
- Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Mediastinal lymph node staging with FDG-PET scan in patients with potentially operable non-small cell lung cancer: a prospective analysis of 50 cases. Chest 1997;112:1480-6. [PubMed]
- Deppen S, Putnam JB Jr, Andrade G, et al. Accuracy of FDG-PET to diagnose lung Cancer in a region of endemic granulomatous disease. Ann Thorac Surg 2011;92:428-32. [PubMed]
- Orlacchio A, Schillaci O, Antonelli L, et al. Solitary pulmonary nodules: morphological and metabolic characterisation by FDG-PET-MDCT. Radiol Med 2007;112:157-73. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung Cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Higashi K, Ueda Y, Seki H, et al. Fluorine- 18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med 1998;39:1016-20. [PubMed]
- Erasmus JJ, McAdams HP, Patz EF Jr, et al. Evaluation of primary pulmonary carcinoid tumors using FDG-PET. AJR Am J Roentgenol 1998;170:1369-73. [PubMed]
- Van Tassel D, Tassel LV, Gotway MB, et al. Imaging evaluation of the solitarypulmonary nodule. Clin Pulm Med 2011;18:274-99.
- Yang P, Xu XY, Liu XJ, et al. The value of delayed (18)F FDG-PET imaging in diagnosis of solitary pulmonary nodules: A preliminary study on 28 patients. Quant Imaging Med Surg 2011;1:31-4. [PubMed]
- Laffon E, de Clermont H, Begueret H, et al. Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun 2009;30:455-61. [PubMed]
- Barger RL, Nandalur KR. Diagnostic performance of dual-time 18F-FDG PET in the diagnosis of pulmonary nodules: a meta-analysis. Acad Radiol 2012;19:153-8. [PubMed]
- National Institute for Health and Clinical Excellence. The diagnosis and treatment of lung cancer (update of NICEclinical guideline 24). Available online: www.nice.org.uk/guidance/index.jsp?action=byID&o=13456
- Schroeder T, Ruehm SG, Debatin JF, et al. Detection of pulmonary nodules using a 2D HASTE MR sequence:comparison with MDCT. AJR Am J Roentgenol 2005;185:979-84. [PubMed]
- Kim JH, Kim HJ, Lee KH, et al. Solitary pulmonary nodules: a comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. J Comput Assist Tomogr 2004;28:766-75. [PubMed]
- Schaefer JF, Vollmar J, Schick F, et al. Solitary pulmonary nodules: dynamic contrast-enhanced MR imaging–perfusion differences in malignant and benign lesions. Radiology 2004;232:544-53. [PubMed]
- Kono R, Fujimoto K, Terasaki H, et al. Dynamic MRI of solitary pulmonary nodules: comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol 2007;188:26-36. [PubMed]
- Mori T, Nomori H, Ikeda K, et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol 2008;3:358-64. [PubMed]
- Zhang X, Ji JX. Parallel and sparse MR imaging: methods and instruments-Part 1. Quant Imaging Med Surg 2014;4:1-3. [PubMed]
- Ji JX, Zhang X. Parallel and sparse MR imaging: methods and instruments-Part 2. Quant Imaging Med Surg 2014;4:68-70. [PubMed]
- Gurney JW. Missed lung cancer at CT: imaging findings in nine patients. Radiology 1996;199:117-22. [PubMed]
- Jaeger S, Karargyris A, Candemir S, et al. Automatic screening for tuberculosis in chest radiographs: a survey. Quant Imaging Med Surg 2013;3:89-99. [PubMed]
- Jeon KN, Goo JM, Lee CH, et al. Computer-aided nodule detection and volumetry to reduce variability between radiologists in the interpretation of lung nodules at low-dose screening computed tomography. Invest Radiol 2012;47:457-61. [PubMed]
- Bogoni L, Ko JP, Alpert J, et al. Impact of a computer-aided detection (cad) system integrated into a picture archiving and communication system (pacs) on reader sensitivity and efficiency for the detection of lung nodules in thoracic ct exams. J Digit Imaging 2012;25:771-81. [PubMed]
- Suzuki K. A review of computer-aided diagnosis in thoracic and colonic imaging. Quant Imaging Med Surg 2012;2:163-76. [PubMed]
- Rubin GD, Lyo JK, Paik DS, et al. Pulmonary nodules on multi-detector row CT scans: performance comparison of radiologists and computer-aided detection. Radiology 2005;234:274-83. [PubMed]
- Gruden JF, Ouanounou S, Tigges S, et al. Incremental benefit of maximum-intensityprojection images on observer detection of small pulmonary nodules revealed by multidetector CT. AJR Am J Roentgenol 2002;179:149-57. [PubMed]
- Suzuki K, Armato SG III, Li F, et al. Massive training artificial neural network (MTANN) for reduction of false positives in computerized detection of lung nodules in low-dose CT. Medical Physics 2003;30:1602-17. [PubMed]
- Li F, Arimura H, Suzuki K, et al. Computer-aided detection of peripheral lung cancers missed at CT: ROC analyses without and with localization. Radiology 2005;237:684-90. [PubMed]
- Suzuki K, Li F, Sone S, et al. Computer-aided diagnostic scheme for distinction between benign and malignant nodules in thoracic low-dose CT by use of massive training artificial neural network. IEEE Transactions on Medical Imaging 2005;24:1138-50. [PubMed]
- Li F, Aoyama M, Shiraishi J, et al. Radiologists’ performance for differentiating small benign from malignant lung nodules on high-resolution CT by using computer-estimated likelihood of malignancy. AJR Am J Roentgenol 2004;183:1209-15. [PubMed]
- Conces DJ Jr, Schwenk GR Jr, Doering PR, et al. Thoracic needle biopsy: improved results utilizing a team approach. Chest 1987;91:813-6. [PubMed]
- Berquist TH, Bailey PB, Cortese DA, et al. Transthoracic needle biopsy: accuracy and complications in relation to location and type of lesion. Mayo Clin Proc 1980;55:475-81. [PubMed]
- Cortese DA, McDougall JC. Bronchoscopic biopsy and brushing with fluoroscopic guidance in nodular metastatic lung cancer. Chest 1981;79:610-1. [PubMed]
- Swensen SJ, Jett JR, Payne WS, et al. An integrated approach to evaluation of the solitary pulmonary nodule. Mayo Clin Proc 1990;65:173-86. [PubMed]
- Henschke CI, Davis SD, Auh Y, et al. Detection of bronchial abnormalities: comparison of CT and bronchoscopy. J Comput Assist Tomogr 1987;11:432-5. [PubMed]
- Jeudy J, White CS, Munden RF, et al. Management of small (3-5-mm) pulmonary nodules at chest CT: global survey of thoracic radiologists. Radiology 2008;247:847-53. [PubMed]
- Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology 2010;255:218-24. [PubMed]